What Is Procenta? What Is Human Tissue Therapy (HTT)? Why Is a Former Employee Mixed Up in This?
This week, I had a colleague send me information about yet another birth tissue company selling a product for use in treating orthopedic injuries. The sales rep claimed to my colleague that this product, called Procenta, was “FDA Approved” for that purpose. Let’s dig in.
What Is Procenta?
Procenta is a placental tissue sold by Extremity Care, LLC. According to the FDA’s Tissue Establishment look-up, it’s NOT manufactured by this company, so this is a sales outfit. The address links back to an office suite in Pennsylvania.
The Message
I got this from a colleague:
“Hey Chris. Got this from a rep. Claim the 361 approval is for all tissue injuries so can be used in Ortho.”
The back and forth further revealed that the sales rep had claimed to this doctor that Procenta was “FDA Approved” for orthopedic indications.
The Plot Thickens
At first, my thought was that this message from a colleague was just an example of yet another idiot sales rep claiming stuff that wasn’t true to push product. However, the more I dug into Extremity Care and Procenta, the crazier things got. First, the company’s Linkedin page had these posts:
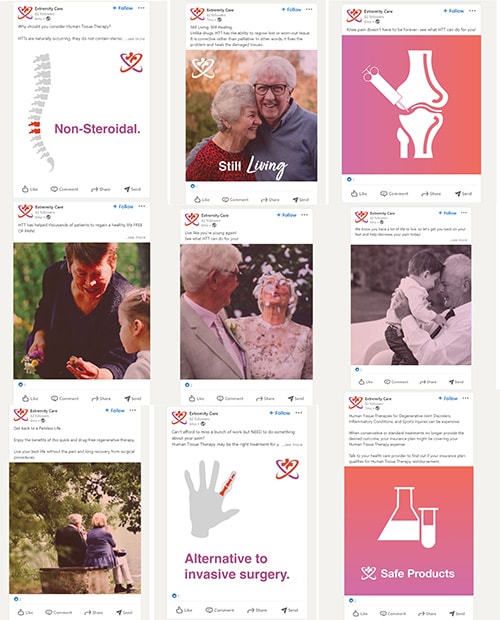
What’s here? Lots of IMHO, dangerous marketing for a birth tissue. Here we learn that the company is selling Procenta for the following medical indications:
- Spine
- General pain
- Knee pain
- Hand and wrist arthritis
- Aging
- Tissue regeneration
The company also claims that HTT (Human Tissue Therapy) is safe and that it’s been used to help thousands of patients in pain. That’s dangerously close to the “safe and effective” moniker that FDA requires full clinical trials and approval to use.
What the Heck Is HTT? Is This Legal?
This was a new one for me. HTT is Human Tissue Therapy, which seems to be Extremity Care’s way of positioning Procenta not as a general wound healing allograft, but as a treatment for pain. While to the reader that may seem like a fine line, IMHO it’s actually a much bigger deal. You see, Procenta has no FDA approval, but merely a 45-minute free registration with no FDA review. Hence, all the company is permitted to say is that they have an allograft for sale, here’s what it is (in this case placental tissue), and here’s what’s in that tissue (perhaps a lab analysis). That’s it. The moment the company crosses the line into treatment claims, that makes Procenta an unapproved drug.
To explain this claims-made regulatory designation, the FDA uses the example of charcoal. You can sell charcoal as a consumer product to fire your grill, but the moment you start to sell that very same charcoal as a treatment for poisoning, that’s a product that requires clinical trials and full FDA approval. To the FDA, what you claim determines the regulatory pathway for what you sell.
Are there any clinical trials that have been published on Procenta? Nope. Any FDA review of those trials and 351 drug approval? Nope.
What Does This Mean?

So why would a sales rep tell my colleague that the product was “FDA approved” for orthopedic use? That could be because the company advertises that it has an FDA/TRG letter on file (red dashed circle above). What’s that? The FDA TRG used to be a service offered by the agency that allowed an interested party to submit a question about the regulatory status of a product. However, that service ended March 31st, 2021. Hence, this likely means that the company submitted a request before that date and got an answer back from the TRG. However, what was in their letter is key here. Meaning, if you look at the company’s website, it’s very vague and uses all of the correct wording that’s regulatory compliant like:
- Our technologies support the repair, reconstruction, replacement, or supplementation of the patient’s own tissue.
- HTTs may only be used in the recipient patient for the same basic function as the cells/tissue functioned in the donor (homologous use).
Hence, if you submitted a request to the TRG using this vague language for a placental tissue, you could expect the agency to state that the free 45-minute registration process (361) would be appropriate. However, if you were to submit that this product is to be used to treat pain, spine problems, joint issues, and general tissue repair, the TRG has shown in other posts that it would consider this a drug that needs full clinical trials and approval. So what exactly does that letter say?
A Call to Extremity Care
To try and get a copy of the letter the company claims to have on file, I called Extremity Care and tried to get in touch with Scott Madden, who is listed online as the President/CEO. I left a message for Scott and will update this blog if I hear back.
Is Procenta Covered by Medicare for Orthopedic Applications?
The sales collateral sent by my colleague shows this complicated-looking document:

This is a Medicare claim form (EOB) that shows that Q4244 was billed and paid for a joint injection (red rectangles above). In looking up this product reimbursement code, this is what I found:

Note that this HCPCS code approval says nothing about orthopedic uses. It’s just about skin wound care, which Medicare does cover, but that coverage is spotty. Can this code be reimbursed by Medicare for orthopedic uses? Well, both Peter Marks, head of FDA CBER and a law firm defending practices that have tried this would beg to differ with Extremity Care. Both have stated unequivocally that this reimbursement is not permitted.
How did this payment happen? Medicare pays all sorts of things it shouldn’t, but when it catches that mistake, it claws back these payments. If it finds that you knew you shouldn’t have billed for that purpose and it paid in error, that’s 10 years of federal prison time for each offense.
Who is Lucina Biosciences?
This Is where following this rabbit hole got REALLY STRANGE. Realize that I began a 4 am and by this time in my deep dive it’s getting close to 7 am. I had performed dozens of Google searches, scoured Linkedin, and read countless documents I found. However, all of that was for Procenta linked to Extremity Care, LLC.
Note that the CMS Q-code approval for Procenta (when used in treating non-healing skin wounds) was obtained by a company called Lucina Biosciences. Huh? Who or what is that? According to an FDA Tissue Establishment look-up, they are the manufacturer of Procenta and are located in Aurora, Colorado. Yikes! That’s about a 40-minute drive from my office. I knew this company name sounded familiar, but when I looked it up online, I was shocked. Lucina lists its Chief Scientific Officer as no other than Ryan Dregalla:
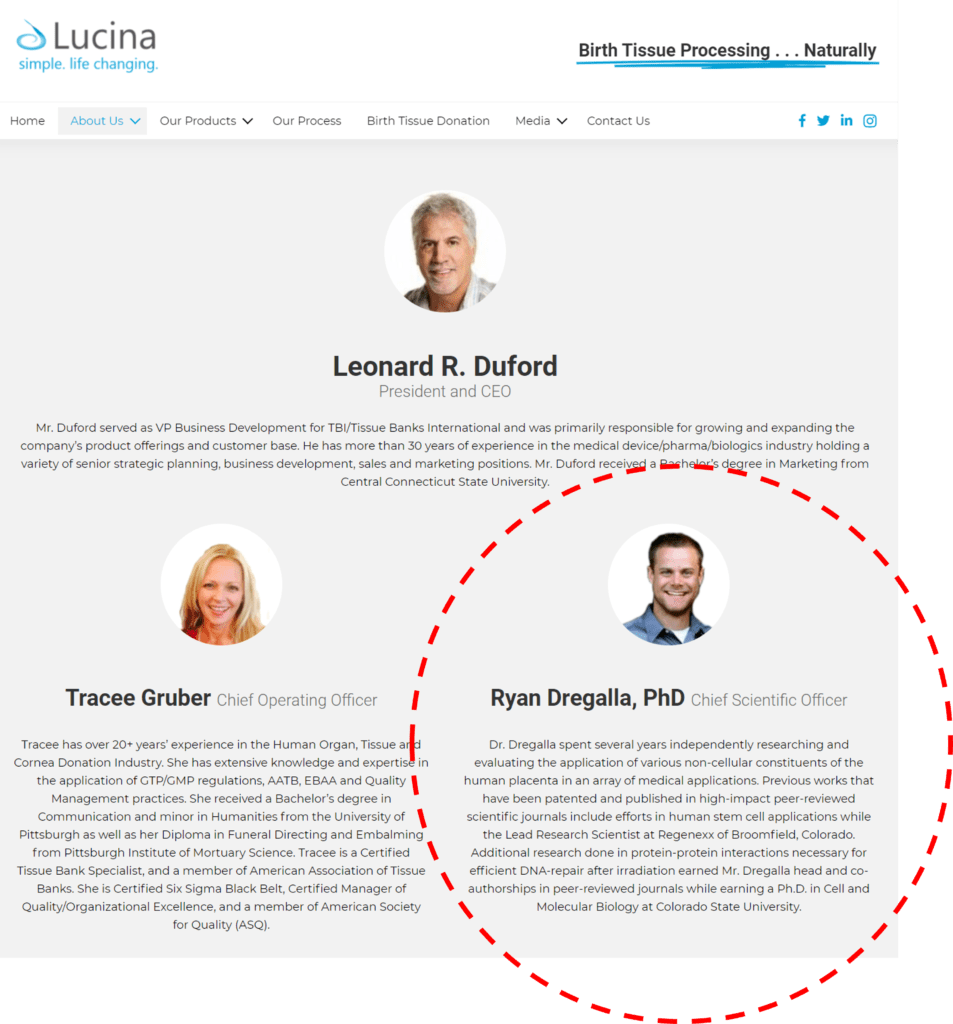
Ryan worked for us many years ago as a lab scientist but hasn’t been associated with our company for some time. Ryan is listed online as a co-founder of Lucina.
I have to say that I was astounded that this rabbit hole led back to Ryan. I tried calling Lucina, but couldn’t reach the company president/CEO, Leonard Duford. If I hear back, I will update this blog. I’ve also pinged Ryan on Linkedin and again if hear back, I’ll be happy to update this blog.
Summary
I have to say that I woke up this morning at 4 am trying to find the energy after a busy week to blog. At first, I messed around with a few topics, but nothing really got me going. When I decided to go down this 4-hour rabbit hole, it looked like just another sales rep that was selling something they couldn’t back up. However, I had no idea that this would be what I found:
- A placental tissue sales company with active orthopedic claims on social media, which IMHO are not FDA compliant
- That purports that it has an FDA letter from the TRG confirming that it can sell its product for orthopedic use (according to my colleague)
- That claims that it can get this product reimbursed for joint injections (based on its own marketing collateral)
- That buys its product from a company co-founded by a former Regnexx employee
Yikes! When I say you just can’t make this stuff up, I mean it! To me, this represents the “new” post-FDA crackdown birth tissues industry. The crazy claims of stem cells and Medicare reimbursements have been largely scrubbed off of websites, but if you look closely enough at the material being handed to physicians and company social media posts, IMHO serious regulatory issues abound.
The upshot? I woke up at 4 am bleary-eyed and trying to get enough gumption and energy on a Friday to get something out. In the end, after working half a day before 8 am, I ended up finding out all sorts of bizarre stuff. And no, IMHO Procenta is NOT FDA-approved nor can you legally bill it to Medicare and get paid thousands of dollars for a simple joint injection.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
