Purchasing COVID-19 Serology Test Kits: A Guide for Physicians (and Patients)
I have spent the last month diving into COVID-19 testing. Mostly from the standpoint of how to protect our employees. However, communicating with my colleagues, I have seen that many are confused by sales reps hawking these tests. In addition, patients are even more confused. Hence, what I’ve found is critical for both physicians and patients to understand.
Molecular vs. Serology Tests
There are two main types of tests commonly being used for COVID-19.
- Molecular
- Serology
Molecular tests use a technology called RT-PCR to look for the genetic material in the virus while serology tests look for antibodies that the body creates to fight the virus.
Molecular Tests
First, molecular tests have many different confusing names such as:
- RT-PCR
- RNA
- Nucleic Acid
In the case of COVID-19, these tests all look for snippets of viral RNA. This is the test where the doctor or healthcare worker sticks a cotton swab up your nose. The purpose is to find coronaviruses and snippets of their specific RNA (the genetic code of the coronavirus). The problem? There isn’t much RNA, so the lab or kit uses an amplification technique called RT-PCR (Reverse Transcription-Polymerase Chain Reaction).
This test, like all tests in medicine, is a two-edged sword:
The Good News:
- This is the only test that can directly detect the presence of the coronavirus
The Bad news:
- The test tends to be positive only in the early stages of the disease (1). This has likely already caused many people who had to wait to get tested to show a negative test result when they really had COVID-19 (false negative).
These tests can be performed through commercial high throughput labs as well as at the point of care (doctor’s office) with devices like:
- Abbott ID NOW
- Cepheid Xpert Xpress
Serology Tests
These tests look for antibodies in the blood that are created by your body to fight the coronavirus. To understand how this works we need to review the types of antibodies you have: IgM and IgG.
IgM is an antibody manufactured by your body in high quantities shortly after a person is exposed to a pathogen like coronavirus and then production declines quickly as the disease progresses. IgG is also produced on the first viral exposure, but not as quickly as IgM. Later in the disease, the antibodies produced are primarily IgG, and they remain in circulation for a prolonged period of time. So think of IgM as the acute sickness antibody and IgG as the immunity antibody.
The Lateral Flow Immunochromatographic Assay
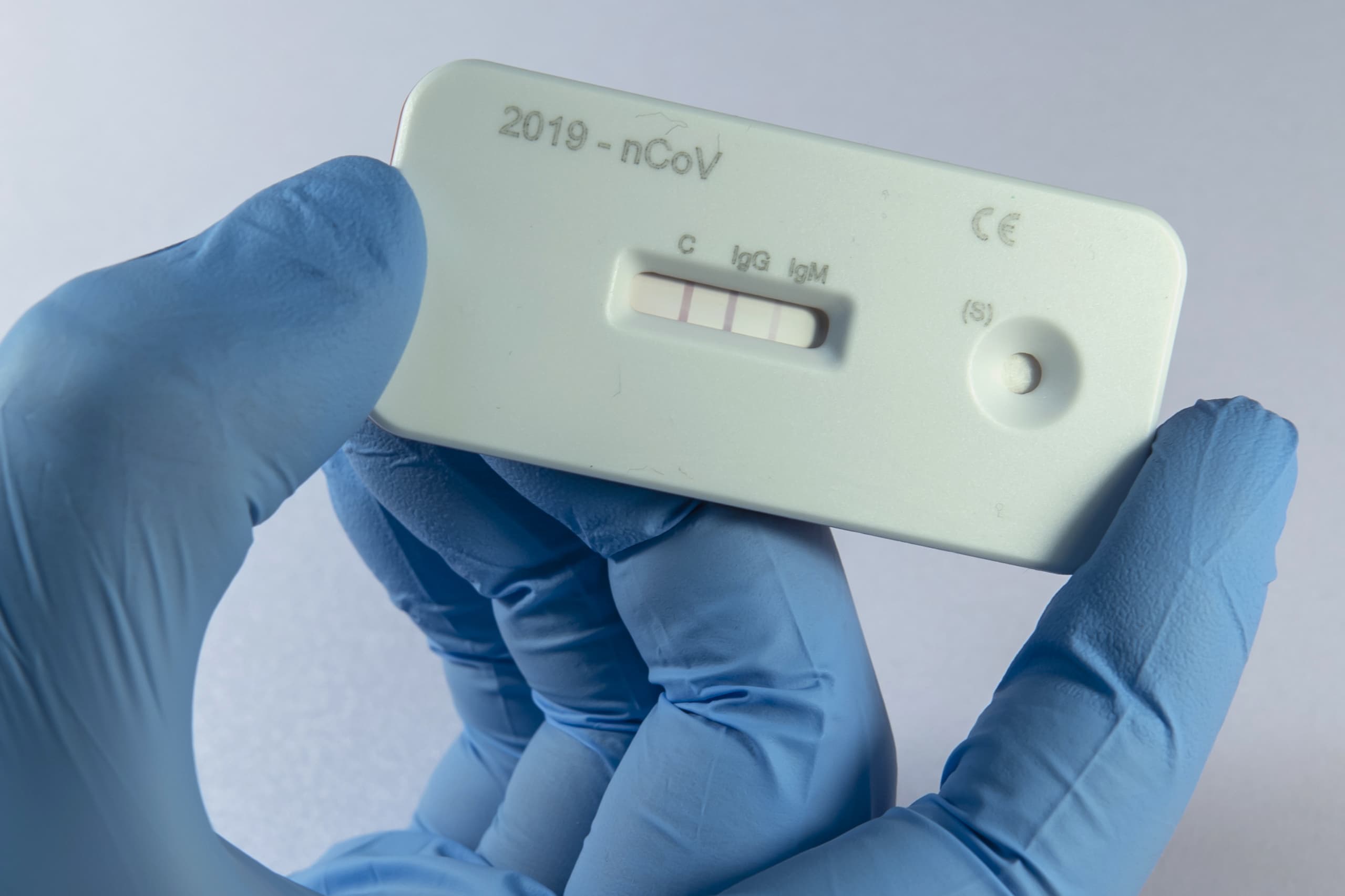
While serology looking for antibodies can be performed in a diagnostic lab as a “titer” quantifying the levels of Ig-M and Ig-G, the most prevalent way that antibodies will be measured during this pandemic will be likely be using a point of care cassette that looks like the one shown here. These allow for rapid 10-15 minute results and look for a specific level of antibodies. How do they work? Let’s dig in.
As shown below, blood is dropped onto one end of the cassette and then a wicking action is used to pull the sample through the device (lateral flow). The first area (conjugate pad) pulls the red blood cells out and then the serum flows to the test lines that have specific anti-antibodies in a colloidal metal (usually gold) and a control line with a general anti-antibody. As the antibodies in the serum bind to anti-antibodies on the lines, they take on a different color.
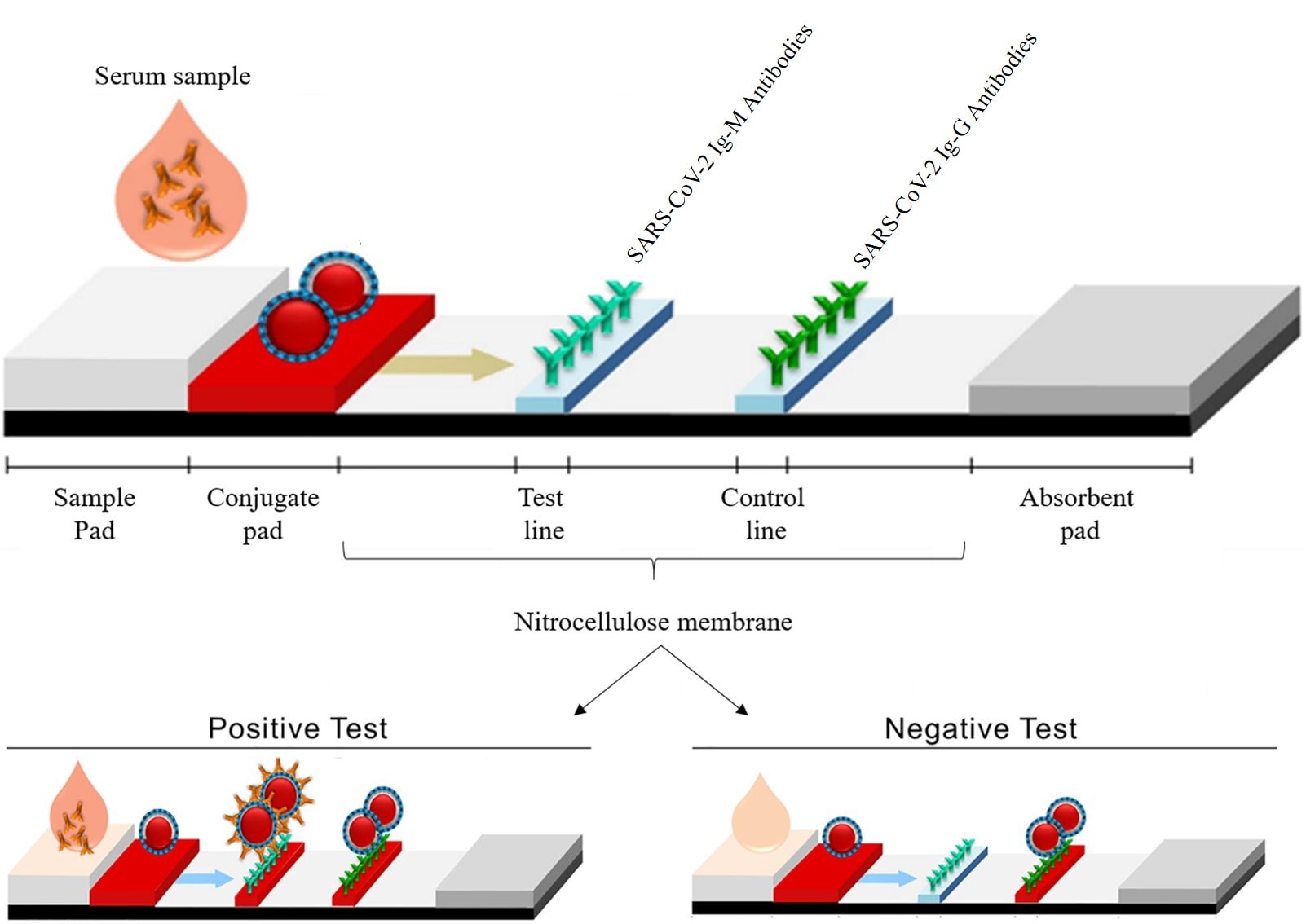
These tests also have positives and negatives:
The Good News:
- This test can directly detect the presence of antibodies
The Bad news:
- The test tends to be positive more in the MIDDLE to LATE stages of the disease (2)
- There can be higher false negatives and positives than with RT-PCR
Threading the Testing Needle
As you can see, neither of these tests is ideal alone. Especially with a disease like COVID-19 with a longer incubation period. For example, one study shows that the median incubation period for COVID-19 is just over 5 days and that 97.5% of people who develop symptoms will do so within 11-12 days of infection (3). In addition, we know through antibody tests that the prevalence of asymptomatic or minimally symptomatic carriers in the general population is quite high (4). Hence, finding people who are positive for this disease will take a massive number of tests.
So what does all this look like when graphed over the course of the disease? See below.
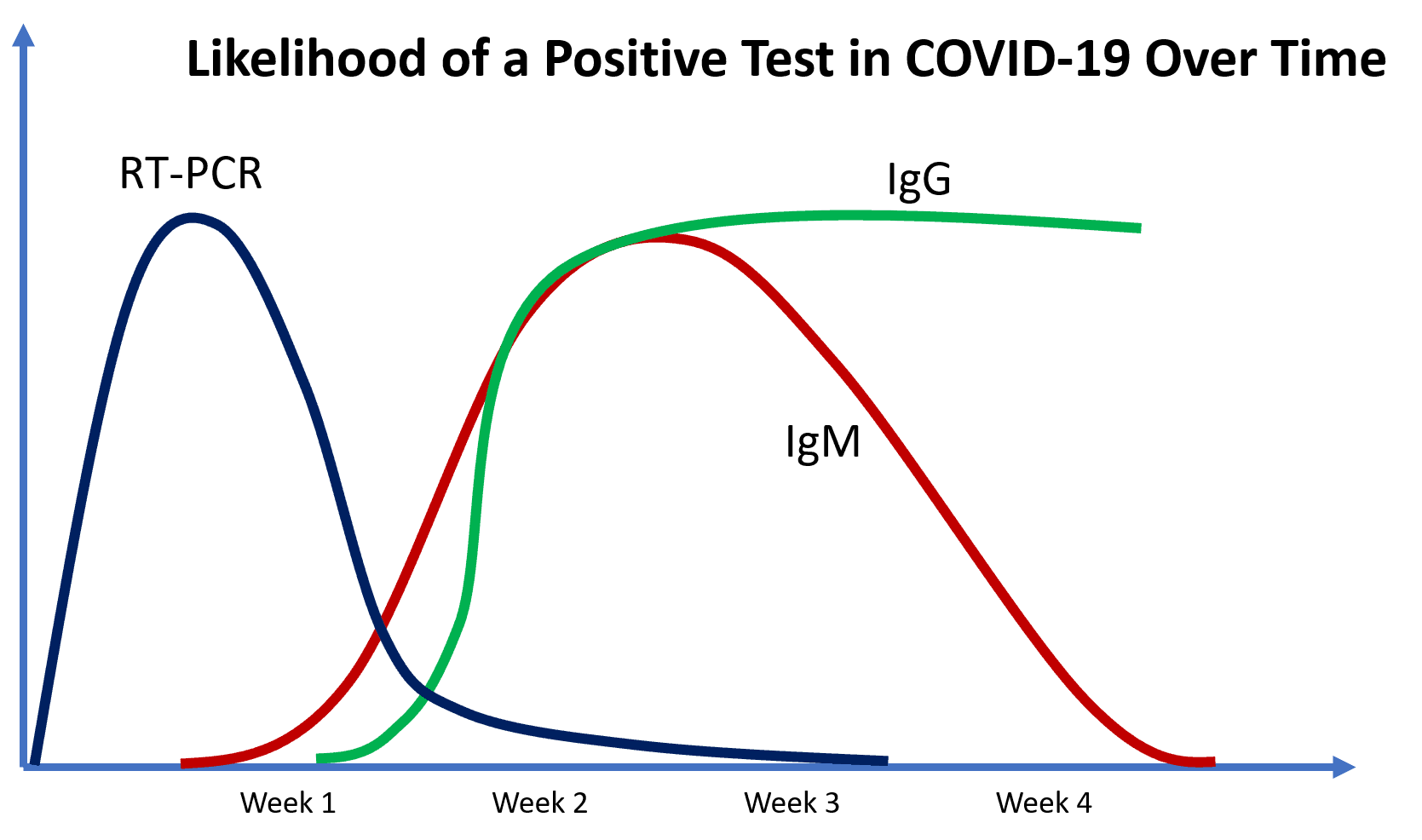
RT-PCR is positive early on, IgM will be positive in some patients in the first few days, but in most not until about a week or more and IgG is positive later in the disease course.
Finding the Right Immunoassay Cassette
Medical product distributors around the country have been gearing up these past few weeks to push antibody cassettes down the throats of any doctor willing to provide his or her credit card. Like all sales reps, each touts their product as the best, but all can’t be right. Also like all sales reps, everything they say must be taken with a big grain of salt. So let’s review what you should be looking for and avoiding.
Understanding the Regulatory Environment is KEY
One of the most interesting things to fully comprehend is that the regulatory status of these antibody cassettes has been an evolving issue. To comprehend this, you need to learn a new FDA term called the “EUA”. This means “Emergency Use Authorization” (5). This was activated at the beginning of the current pandemic and in terms of these tests, it permitted manufacturers to import tests that had approvals in other countries for sale in the U.S. In order to be in compliance, the manufacturers needed to “register” their products with FDA (which is not an approval, merely a free online form to fill out).
Finding the EUA Registration
The first thing you need to make sure of is that the test you plan to buy has been registered with the FDA. The tests that have been registered can be found at this link. THIS IS NOT AN FDA APPROVAL!
The Sticky Wicket-EUA Approval
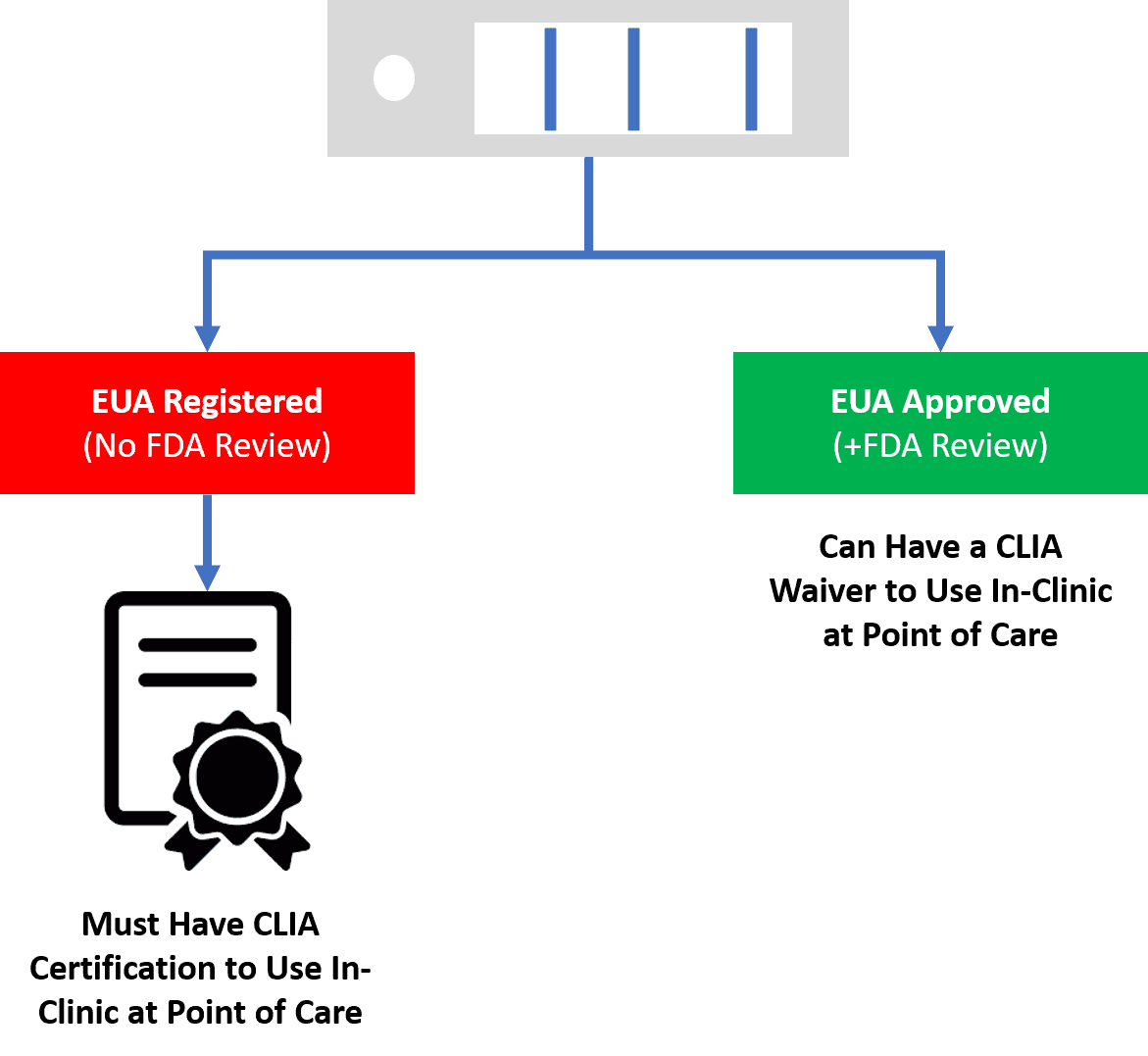
The FDA on its webinars for industry has now clearly stated that if a doctor’s office wants to use a test that only has a EUA registration than that physician needs to have a CLIA lab certificate. THIS IS VERY DIFFERENT FOR POINT OF CARE TESTS, WHICH USUALLY ONLY REQUIRE A CLIA WAIVER. A doctor’s office may use a point of care test which has a EUA Approval with a CLIA waiver.
Realize that a CLIA waiver and certificate are VERY different. First, CLIA stands for the Clinical Laboratory Improvement Amendments and it’s the U.S. government process that oversees diagnostic labs. A CLIA certification for complex testing is a big deal and most physician offices wouldn’t qualify. A CLIA certification for lower-level tests is something some medical practices have, but to get one in place would take months. Hence, most doctor’s offices have a CLIA waiver that allows them to run point of care tests that are certified to be easily run in the clinic.
Given that very few of the tests being sold by sales reps have a EUA Approval which is an intensive review by the agency of the test accuracy after reviewing thousands of sample test results, that means that most physician offices will not be permitted to use most of the tests. The sales reps, in my experience, are actively deceiving physicians on this point or just don’t have any idea of what the regulations really are at this juncture.
Getting EUA Authorization-Betting on the Right versus the Wrong Horse
Hence, if you’re the average physician’s office who doesn’t have a CLIA lab certification, you should be looking for one of the rare tests that have a full EUA Approval where FDA has reviewed the accuracy of the test. Given that this is a fluid situation in a public health emergency, how can you be confident that the test you chose will eventually get this special approval from FDA?
First, the FDA has telegraphed it’s punches here. They have said that they will not consider EUA Approval test submissions that don’t have at least 1-2,000 results. Hence, ask to see the IFU (Instruction for Use or package insert). On that document, it lists the validation methods and numbers. If that lists the results of 40-250 tests (which is the common range if have seen while reviewing many IFUs these past few weeks), then it’s unlikely that the test will get a EUA Approval unless the manufacturer submits more data. This doesn’t necessarily mean that this is a bad test, but it may not be a test that you will be permitted to use long-term! Meaning that it’s likely that the FDA will exercise some regulatory discretion here for the next month or so, but after that, it’s more likely that they will begin cracking down after the testing log-jam breaks open.
Test Accuracy
Obviously, every physician wants an accurate test, especially when most of these tests will be used to ID asymptomatic carriers and patients with immunity. One of the biggest issues with serology cassettes is understanding how the test was vetted. Let’s dive in.
Test Validation
Test validation information should be on the IFU (Instructions For Use) package insert. This data usually comes in two types:
- Clinical Agreement Study
- Cross-reactivity
Let’s dig in.
Clinical Agreement Study
This means that the serology test was compared to actual patients being treated with a clinical and lab diagnosis of COVID-19. This is the classic sensitivity and specificity of the test looking for false-negative and false-positive results. This data is also often used to determine when the test is first shows positive in the course of the disease.
To review basic testing accuracy terms:
- False Positive-When the tests shows positive but the patient doesn’t have the disease
- False Negative-When the test shows negative, but the patient does have the disease
The goal here is to reduce false positives and negatives. Hence, the lower these rates are the better. However, there’s also the idea of cross-reactivity, which can be a huge issue here.
Cross-reactivity
This means that the test will show positive with another common virus or other factors due to shared proteins between it and the coronavirus (a false positive). The common ones I’ve seen tested include influenza A and B, RSV, Adenovirus, and a few others that are not respiratory-related. I have yet to see any serology test that flunked this basic test of cross-reactivity.
However, there are many coronaviruses out there and many share similar proteins that may cause the serology cassette to produce a false positive. This is where the rubber meets the road for these cassettes. Some cross-reactivity can be observed with:
- SARS-CoV-The coronavirus that caused the 2003 global outbreak. No longer circulating, so not an issue.
- Rheumatoid Factor-This could be a huge issue if a patient is suspected of having rheumatoid arthritis.
- MERS-CoV-The Middle East SARS virus that kills rapidly, but doesn’t spread widely because people are usually in the ICU while infectious. So this is a non-issue in this pandemic because if you have the MERS virus, you have bigger problems.
- HKU1, NL63, OC43, or 229E-Coronaviruses that cause the common cold.
Hence the biggest cross-reactivity issues are obviously that the test could show positive for a common cold type coronavirus infection.
Disclaimer for Using a EUA Registered (and not Approved) Products
Because the issues noted above haven’t been resolved by FDA, in using an FDA EUA registered test (no EUA Approval), you need to include the following required information in a testing consent:
- This test has not been reviewed by the FDA.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the
virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these
individuals. - Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as
coronavirus HKU1, NL63, OC43, or 229E.
Interestingly, I have seen cassette serology tests being sold by medical sales reps that do not include this cross-reactivity data. In fact, one American product being sold widely doesn’t even include a statement about cross-reactivity with common coronaviruses despite having no EUA approval. Hence, you must look at the IFU!
Bottom Line
After looking at these products now for weeks, here’s what I have learned:
- Choose a product that is on the EUA approval track. That means that the company is working with a university to submit the results and data on thousands of tests. These will be more expensive.
- There are VERY FEW tests being sold right now that have EUA approval or are legitimately on this track. So do your homework and don’t trust what sales reps tell you. Ask to see the EUA Approval submission document.
- If you use a product that only has a EUA registration, make sure your consent warns patients of possible false positives and negatives. If you have a patient suspected of COVID-19 that has symptoms and a registered cassette shows negative, then you need to get RT-PCR testing. Similarly, if you have an asymptomatic patient that shows positive, you should confirm with RT-PCR testing.
The upshot? While I think serology cassettes will prove very useful in helping us solve this pandemic by rapidly identifying patients with antibodies, there are issues with these products in this early phase that need to be vetted by the physician. Hence, buyer beware!
__________________________________________
References:
(1) Wikramaratna P, Paton R, Ghafari M, Lourenco J. Estimating false-negative detection rate of SARS-CoV-2 by RT-PCR. medRxiv 2020.04.05.20053355; doi: https://doi.org/10.1101/2020.04.05.20053355
(2) Pan Y, et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect. 2020 Apr 10. pii: S0163-4453(20)30175-4. doi: 10.1016/j.jinf.2020.03.051.
(3) Lauer SA, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Annals of Internal Medicine, 2020; DOI: 10.7326/M20-0504
(4) Bendavid E, et al. COVID-19 Antibody Seroprevalence in Santa Clara County, California. medRxiv 2020.04.14.20062463; doi: https://doi.org/10.1101/2020.04.14.20062463
(5) U.S. FDA. Policy for Diagnostic Tests for Coronavirus Disease-2019 during the Public Health Emergency. https://pellecome.com/wp-content/uploads/2020/03/IIE-COVID19-Guidance.pdf Accessed 5/2/20.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.