Regenexx Knee Arthritis Data: Are the Patient Results We Show “Research Grade”?
We have been performing stem cell procedures to treat knee arthritis for 15 years. We’ve published many papers on the topic and have almost 10,000 patient results listed on-line. However, is that patient results data the same as real research data? Let’s dig in.
Knee Stem Cell Procedures to Treat Knee Arthritis
We’ve been helping patients avoid knee replacement by using their own bone marrow stem cells since 2005. In that time, we’ve published MRI case reports, various large case series, comparison trials, dosing analyses, and a randomized controlled trial (1-4). Put all of that together with one of the world’s longest-running clinical experiences on the topic and we’re pretty convinced this stuff works. To learn more about this procedure versus knee replacement, see my video below:
Registry Data
Regenexx tracks its patients in a registry. That’s a database that pings patients intermittently to record their outcome and any complications from the procedure. That makes us pretty unique, as few physicians who operate in this field take the time and the expense to do that. In fact, if you go to the “Results” tab above at the top of this webpage and then click on “Patient Outcome Data”, you’ll be able to access the most recent registry outcome information (updated once a month). A running man graphic will come up and you just click on a body part to begin:
If you click on “Knee” and on “Osteoarthritis” and finally on “Overall Joint Improvement”, you’ll get a graph that looks like this:
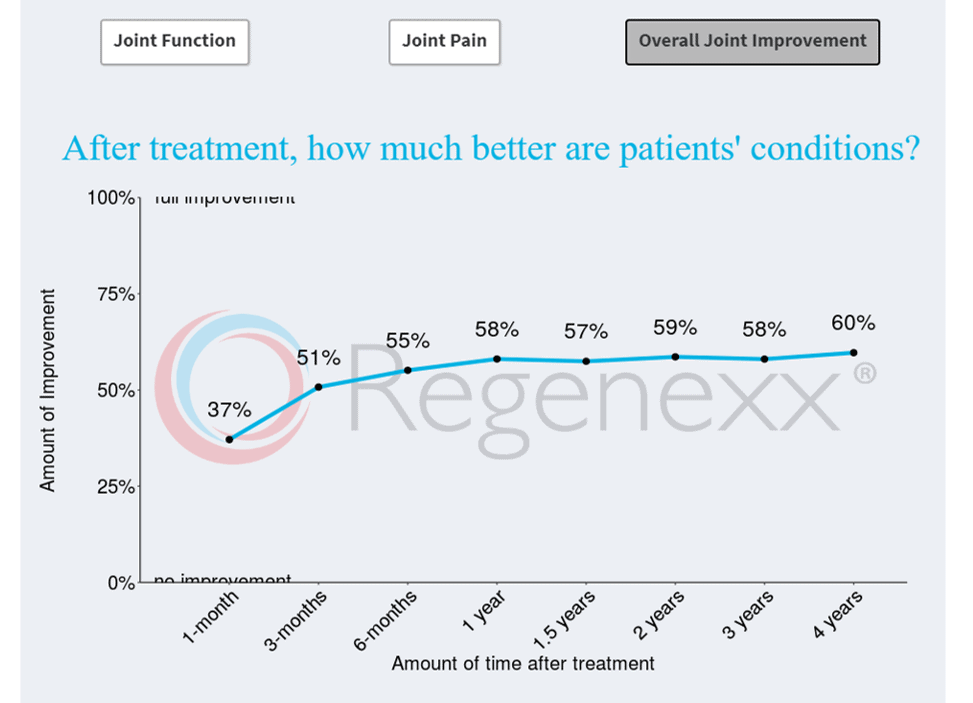
While this looks great and represents information reported by thousands of patients, can you trust this registry data? Meaning, the graph above shows that on average, these patients report about 60% (59%) improvement at 2 years. Is that number influenced by the fact that asking thousands of patients to fill out questionnaires will result in many that never respond? Let’s dive into the issues.
The Upside and Downside of Registry Data
The upside of a registry is that it can collect massive amounts of data on a massive number of patients. For example, as of this writing, we have 9,885 patients who had the Regenexx knee stem cell procedure being tracked in the registry. The downside of a registry is that follow-up rates are usually in the 40-60% range. That means that many patients will eventually refuse to answer the questionnaires you send them. The question then becomes, does all of that lost data (i.e. patients that refuse to answer) skew the outcome?
For example, you could argue that a patient who doesn’t answer is probably happy with their result, and hence if you had all of the patients answering that the outcome would be even better. You could also argue that patients who don’t answer are possibly unhappy with their result and if you added these patients the outcomes would look worse than the registry portrays. Finally, you could argue that adding back in everyone who failed to answer would be a wash and that the registry data is mostly accurate.
So which is it? We tried this experiment a number of years ago with 100 patients. We got to almost 100% response in this group to see if that information was different than the registry data. The good news was that it tracked very closely. Today I’ll run this same experiment with a new larger dataset from a research project with higher follow-up rates.
The Research Dataset
This data was collected as part of a separate research study testing whether we could predict the outcome of our stem cell procedure based solely on the chemical composition of the fluid in the knee. Because this data was collected as part of that research, our research coordinator hounded these patients to make sure as many as possible answered their questionnaires. Meaning, on average, we got about twice as many people to answer these questionnaires compared to the response rates in the registry. For example, the one-year response rate here is 84%. So how do the two stack up? The registry versus the research data?
Below I have overlaid the two data sets. The black circles and the black line is the SANE (Single Assessment Numeric Evaluation) score for the research subjects. This is an overall patient report of improvement. For example, if the patient said their knee was 50% better at that time point (1, 3, 6, 12, 18, or 24 months) then the SANE score is 50. For the registry dataset, since patients answer at different rates and since new patients are being added all the time, the patient numbers reporting at any given point are in the 3-5,000 range. For the research dataset, for the same reasons, the patient numbers are in the 200-350 range.
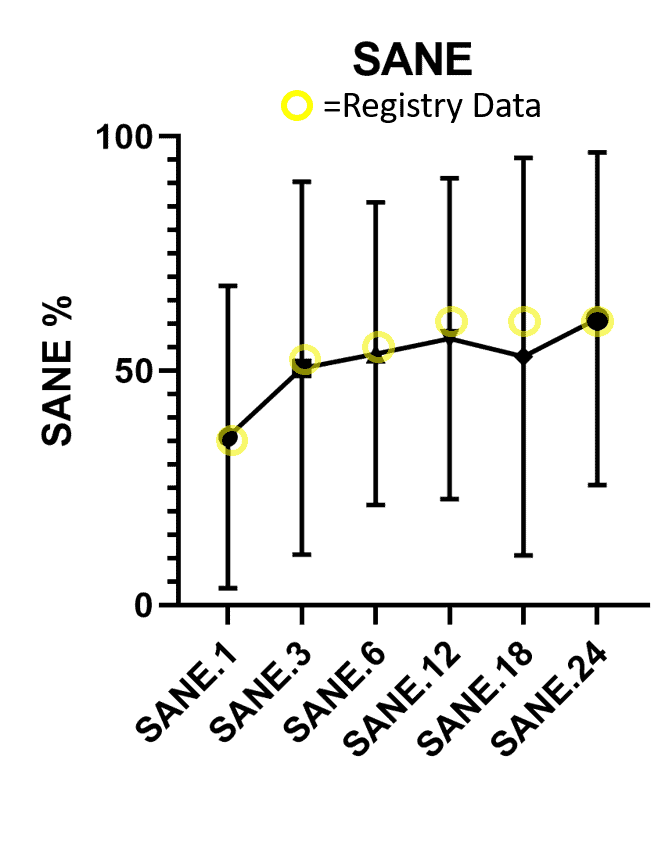
As you can see, the yellow circles (the registry data with thousands of patients and more patients that never answered) track very closely to the research data with higher response rates (black shapes). That argues that the patients that never answer the registry questionnaires are a wash. The ones who are unhappy with their result are equally opposed by patients who are doing well.
Pretty Amazing Results
Since this research dataset of patients with knee arthritis has a higher response rate, what else can we learn? First, a very high percentage of the patients in this dataset were knee replacement candidates at the time of their treatment. Hence, what percentage opted for a knee replacement despite getting a Regenexx procedure? About 10%. That’s consistent with the last time we looked at this information which showed that the knee replacement rate despite treatment was 13%.
At one and two years after the procedure, what percentage of patients had 50% improvement or more? At 1 year that was 70% and at two years it was 73%. If we increase that to more than 70% better, what fraction attained that benchmark? That’s 52% and 57%. At two years what fraction of these patients were 100% better? That’s 13%. The data table is below:
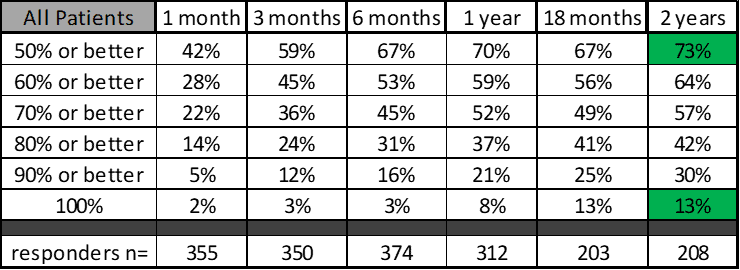
If we run statistics on these numbers, which time points show statistically significant improvement? For example, if we compare the 1-month result to the 12-month result (“1 v 12” below). That table looks like this:
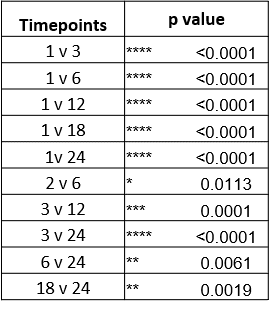
This shows that most of these comparisons show statistically significant improvements.
The upshot? It’s really cool to see our registry data tracking with the research data. There’s always the concern that registry data isn’t the same as a research study. I think our data argue that when you pull up our Regenexx registry data, you can trust that it’s “research-grade” information!
___________________________________
References:
(1) Centeno C, Sheinkop M, Dodson E, et al. A specific protocol of autologous bone marrow concentrate and platelet products versus exercise therapy for symptomatic knee osteoarthritis: a randomized controlled trial with 2 year follow-up. J Transl Med. 2018;16(1):355. Published 2018 Dec 13. doi:10.1186/s12967-018-1736-8
(2) Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int. 2014;2014:370621. doi: 10.1155/2014/370621
(3) Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008 May-Jun;11(3):343-53. https://www.ncbi.nlm.nih.gov/pubmed/18523506
(4) Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet Disord. 2015;16:258. Published 2015 Sep 18. doi:10.1186/s12891-015-0714-z

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.