Umbilical Cord MSCs Sales 2.0: Using the Expanded Access Program?

You have read here many times about the fraudulent claims of umbilical cord tissue vendors that they are selling products with millions of young and vital mesenchymal stem cells. That was followed by the FDA crackdown last year and all seemed quiet for a while. However, now we’re seeing a new business plan emerge that uses an FDA program meant to get potentially life-saving drugs to the terminally ill to bypass clinical trials and FDA approval. Let’s dig in.
Wharton’s Jelly products 101
The umbilical cord contains a jelly-like substance called Wharton’s Jelly (WJ). This provides structural support to the cord and has many components, with rare mesenchymal stem cells. How rare? Between 1 in 300 cells to 1,609 cells (1,2). Meaning that the total percentage of MSCs is small, approaching 0.3-0.06%.
When you take an umbilical cord from the labor and delivery department right to a lab and then digest the Wharton’s Jelly using collagenase to free up cells and then place that product in a tissue culture you can get mesenchymal stem cell (MSC) colonies to form. Based on this fact, there have been wild claims about commercial WJ products that are being sold to doctors containing millions of viable and functional MSCs.
The Problem with Commercial WJ Products
What happens when you take an umbilical cord, save it in a fridge in a hospital until someone picks it up, drive it across town, package it, place it on a plane, drive it across town again in a FedEx truck, put it back in a fridge, and then at some later time process it? Given that this is what’s often done to the umbilical cords that make up commercial WJ products, this cell torture causes problems. Our lab research team first began to notice back in 2014 that birth tissue products, despite wild claims of viable live stem cell content, didn’t actually have any live and functional MSCs. That lead to a research paper published in collaboration between CSU and our research team that showed that WJ products that all claimed to have plentiful MSCs didn’t have any that survived past a few hours. Basically, the cells were all dead or apoptotic (dying)(3).
The Viability Problem
The WJ products I describe above come frozen, often at -80C. This causes yet another problem. The first, as described above is that the umbilical cord itself makes a long journey that causes cells to die or become apoptotic. The second problem is that properly thawing frozen cells is a process by which you slowly warm them and place them in a recovery culture for several days. However, the doctor’s offices that buy these products have no ability to do this, so they warm the bottle quickly. Hence any cell that wasn’t already dead or dying perishes in this shock thaw.
Accurately measuring cell viability is usually an exercise in scientific bait and switch for birth tissue vendors. The most common way this is done by WJ manufacturers is using a Trypan Blue dye. The problem is that all this tells you is if the cell was alive when you stained it. It doesn’t tell you if the cell was alive but sick and dying. As a result, when you stain the cells that come out of WJ commercial products you can see “viability” results of 60-85%. However, more sophisticated stains that require more sophisticated lab equipment show that almost all of these cells are in the process of dying (apoptotic). Hence, when you try to culture these cells to prove that they will survive in the body, those cultures fail.
Take for example these pictures below (CFU-f assays from our lab), where the purple dots are viable MSC colonies and the all-white means all dead cells:
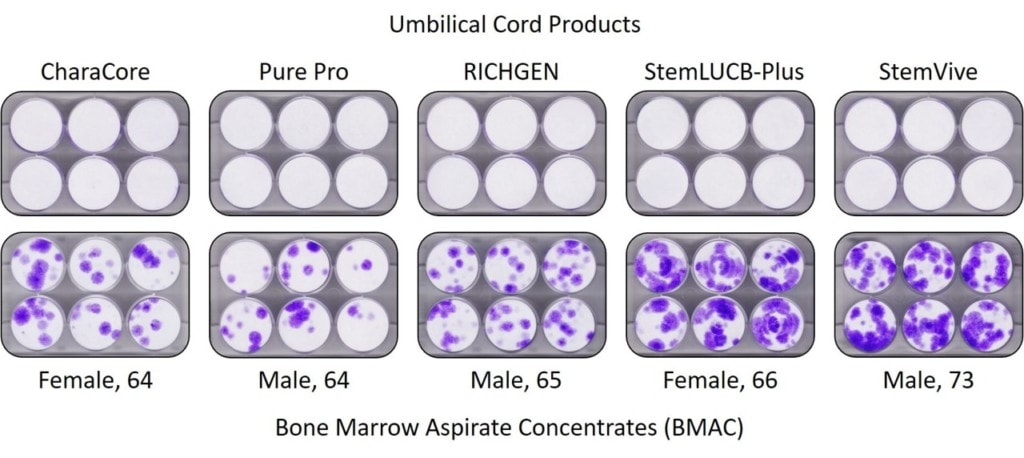
All of the commercial WJ products tested that claimed to have loads of visible MSCs showed all dead cells (all white-upper row). On the other hand, bone marrow from elderly people (lower row) showed viable MSCs (purple dots in the row below).
Dramatic Regulatory Changes
Then the agency ended its discretionary period. Meaning that it had told violative companies that it expected all of them to have FDA drug approval for these WJ products by a certain date. Hence, many smart companies folded shop at that time, not wanting to spend the tens to hundreds of millions of dollars that it takes to run clinical trials and obtain FDA approval.
Then the FDA recently dropped “the big one” by tightening the medical indications for umbilical cord products when used as a registered tissue rather than a drug. That is now:
- “serving as a conduit (for umbilical cord)”
Hence, unless you’re treating fetuses with this stuff, there is no FDA-approved use for WJ tissue products. Meaning these products can no longer legally be sold or used in orthopedic injuries or diseases without full clinical trials and formal approval as a new drug. Given that no manufacturer has ever done that, all of these products being currently sold as tissues for orthopedic use are now technically unapproved and illegal drug products.
The New Business Plan
You might think that all hope is lost for any WJ manufacturers out there trying to sell this stuff for orthopedic use to doctors like me. However, you would be wrong. A case in point is this message that I got on Linkedin from a WJ manufacturer:
“Our business model works through expanded access towards treatments with excellent high quality partnering clinics. We aim to work from individual treatments to a group treatment model for various indications that can go up to 1,000 patients in the group model for each indication. Ultimately working towards approved biologic license.
We are seeking experienced regenerative partners to work together and that’s exactly why I thought specifically of you and your expansive team with an incredible reputation.
Per FDA requirements, treatment clinics submit as the treating partner and we could submit as the manufacturing sponsor for Expanded Access Treatment IND.
Love to chat more about this model and our USA manufacturing facility and put you in touch with our CEO.”
Let’s unpack what’s being said:
Is This Legal?
NO. Note the sentence, “Ultimately working towards approved biologic license.” A BLA is what a drug sponsor would receive AFTER successful clinical trial results are approved by FDA. None exists for this WJ product. Can you sell your product before you have a BLA. NO. If you do, then you’re breaking federal law.
What Is Expanded Access?
The FDA’s “Expanded Access” program (aka “compassionate use”) allows terminal patients to receive access to a drug that’s still in development. Meaning there is an IND in place and the drug is still being tested in clinical trials where the large-scale safety and efficacy are still unknown. However, this program is very specific and has high hurdles that must be jumped:
- Patient has a serious disease or condition, or whose life is immediately threatened by their disease or condition.
- There is no comparable or satisfactory alternative therapy to diagnose, monitor, or treat the disease or condition.
- Patient enrollment in a clinical trial is not possible.
- Potential patient benefit justifies the potential risks of treatment.
- Providing the investigational medical product will not interfere with investigational trials that could support a medical product’s development or marketing approval for the treatment indication.
NONE of the patients we treat, since we’re focused on orthopedic and spinal problems that cause pain and disability fits within the definition of a life being threatened by a serious disease. In addition, given that autologous PRP and Bone Marrow Concentrate are permitted to be used for many of these orthopedic conditions and have an expanding research base showing efficacy, other treatments are available. You can also bet that the FDA would count things like hyaluronic acid or corticosteroids as valid alternative treatments for knee arthritis. Hence, using the FDA’s Expanded Access program IMHO is a non-starter for this WJ company.
1,000 Patients per Clinical Indication
Remember that the purpose of the expanded access program is to allow terminal patients to try drugs that are not yet fully FDA approved. Our WJ product fails this test when used to treat knee arthritis. In addition, you would want (as is suggested here) each of those treated patients to collect data that counted towards an FDA-approved clinical trial. That’s the game plan indicted by this statement, “Ultimately working towards approved biologic license.”
So how much would it cost to treat 1,000 patients per clinical indication like knee arthritis and 1,000 patients for shoulder rotator cuff tear in this way? It would be “MM” or “Many Millions”. First, you need to hire a clinical research organization to track all of this data. In addition, the clinical sites at universities and private practices expect to be paid at much higher rates for these cases where research data must be collected. For example, the current cost for a clinical trial is about $41,000 per patient. So treating a thousand patients would be tens of millions of dollars per clinical indication. Meaning that you might as well just perform an FDA-approved clinical trial and get it over with.
You could try to get patients to pay for all of this, but everyone I have ever interviewed who has tried to get cost reimbursement, after speaking with FDA, has decided not to proceed. It’s just too costly and burdensome. In addition, it adds all sorts of issues. How do you handle placebo or sham care? Will patients who realize that their part of a clinical trial with strict inclusion and exclusion criteria pay for care?
Finally, there’s another big issue. The expanded access program was designed for one-off approvals for patients who were terminally ill. It’s apparently also possible to get larger blocks of patients approved. However, what do you think will happen when the agency finds out that you’re using the expanded access program in lieu of a formal clinical trial to treat knee arthritis patients? Or when they find that you used it as an advertising program to try to get doctors to buy your WJ product? IMHO they will quickly shut this down.
As you can see above, this new game plan of using the expanded access program to bypass FDA clinical trials is not tenable. That’s doubly true for orthopedic applications which are not life-threatening. Hence, if you get this sales pitch, my advice is to run and get as far away from this poorly conceived plan as possible.
_______________________________________________________
References:
(1) Sarugaser R, Lickorish D, Baksh D et al. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells 2005; 23: 220– 229.
(2) Lu L, Zhao Q, Wang X et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006; 91: 1017– 1026.
(3) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Oct;49(12):3404-3413. doi: 10.1177/03635465211031275. Epub 2021 Aug 16. PMID: 34398643.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.