Using Birth Tissues to Treat COVID-19? Injecting a Cytokine Storm
Many providers who use orthobiologics have used various birth tissues to try to help patients heal orthopedic injuries. However, many have also discovered that they’re a two-edged sword. On the one hand, they can enhance healing but on the other, they can also stoke serious inflammation. Now some providers are advocating their use to treat COVID-19. Today I’ll share some data that shows why this is likely the equivalent of Russian Roulette.
Birth Tissues 101
Birth tissues have been used for decades in many types of surgeries. For example, neurosurgeons would often tap the OB ward for pieces of amniotic membrane to replace the covering of the spinal cord called the dura. Ophthalmologists have also used these tissues in corneal repairs. Then about a decade ago, some companies began selling these tissues to promote wound healing. Shortly thereafter, a few companies with no scruples began to claim that their birth tissue products had stem cells, which turned out to be fraudulent as later research showed this to be untrue (1-3).
Our Use of Amnion
In select patients, we have noted that since amniotic membrane products contain growth factors that can promote tendon healing, they can sometimes be added to PRP injections to promote tendon healing. PRP stands for platelet-rich plasma and this is when the doctor takes the patient’s blood and concentrates the healing platelets which also have growth factors. For example, when a patient has a rotator cuff tear, PRP injection precisely into that area can often save them from needing more invasive surgery.
Hence, when we decided to source a single amnion vendor for our entire network of more than 70 U.S. clinics who can help patients avoid shoulder surgery, we used our advanced lab capabilities to test many different products for growth factor and cytokine levels. What we found turned out to be good news or bad news for COVID-19 patients who are now getting offered this stuff as treatment. In fact, what we found could be a dangerous game of Russian Roulette.
The Inflammatory Cytokine Russian Roulette
If you recall from other posts, many hospitalized COVID-19 patients end up with a dangerous “cytokine storm” of inflammation in their lungs which causes respiratory failure. Of the many ways that this could be treated, one of the purposed therapies is a specialized stem cell product that’s made in a lab. Because of the fiction that amniotic tissues contain stem cells, some manufacturers of these birth tissues have taken the position that patients should be given their birth products as a therapy to prevent or treat COVID-19.
There’s just one serious problem. Our research shows that some of these tissue products have much higher levels of natural inflammatory cytokines. Basically asking for trouble in treating COVID-19.
Our Testing
Given that we have treated many patients with rotator cuff tears and helped them avoid surgery, we began testing amniotic products to help our providers bridge the gap between a patient that just needs a precise PRP injection versus a full stem cell injection procedure. Amnion plus PRP can fill that doughnut hole nicely in our experience. What were we testing for?
First, while every other provider in the country that offers orthobiologics would be at the mercy of the sales reps in buying the right amniotic product, Regenexx has it’s own university-level lab in which to test products. Hence, we began testing different products and different lots of the same product for growth factors like FGF which are known to help tendon cells heal. We also began to notice some disturbing trends in other growth factors associated with inflammation.
Gasoline on a Bonfire
Anyone who has injected patients with amniotic tissue products knows that sometimes they can stoke up severe swelling and inflammation in patients. I call this the gasoline on a bonfire effect. When this data began to come in from our testing we began to see the likely reason why this happens. For example, take the samples we tested below:
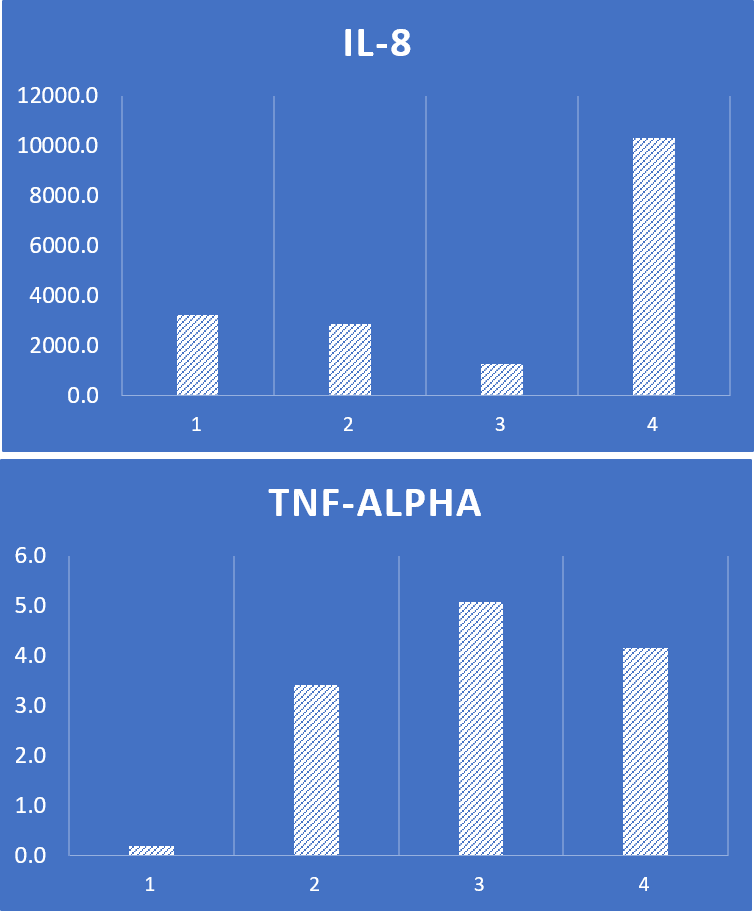
These are levels of two inflammatory cytokines in various lots of the same product we tested. Note that sample 1 above is low in both IL-8 and TNF-a. However, lots 2,3, and 4 are high in these “gasoline on a bonfire” inflammatory chemicals.
Amnion and COVID-19
So what would happen if you tried to inject a product like this intravenously into a COVID-19 patient? It would be like throwing gasoline on that cytokine storm bonfire. It could just as easily prime the patient for more severe COVID-19 or if they were already sick, it could put them in the ICU on a ventilator.
The upshot? As our research shows, different lots of the same product contain vastly different levels of inflammatory cytokines. While in a rotator cuff tear patients that may mean more swelling in the area, for a patient trying to prevent or treat COVID-19, that may be enough to cause serious harm. So please avoid using these birth tissue products to protect or treat this disease.
___________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.