What Is Differentiation, Dedifferentiation, and Transdifferentiation?
There are a few critical things you need to know about stem cells that most doctors and patients considering them don’t know. That’s how differentiation, dedifferentiation, and transdifferentiation work. Let’s dig in!
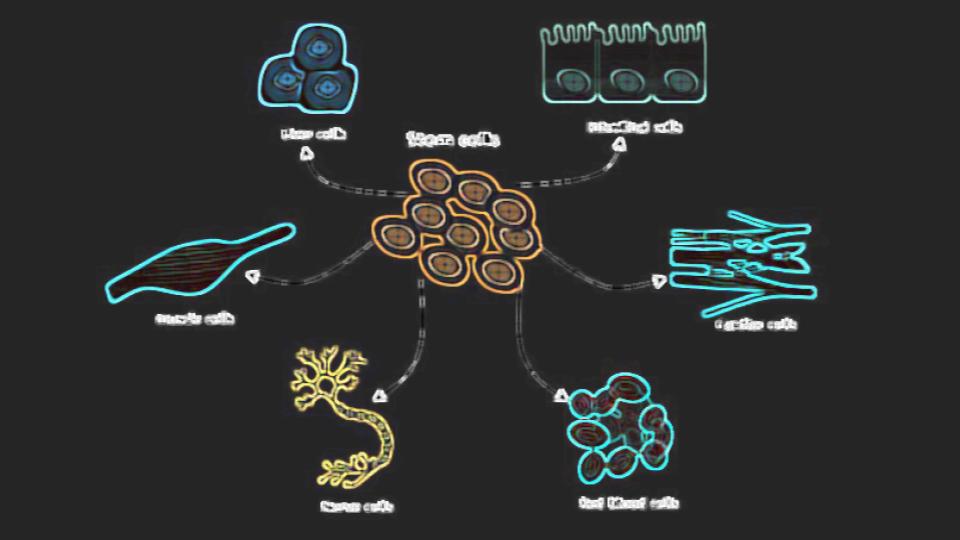
Stem Cells 101
Stem cells are present everywhere in our bodies and act as repairmen. You can’t go a day without damaging your cells a bit and with some activities more than a few cells are damaged. How do these cells get replaced?
A local stem cell in that damaged tissue is usually the key to tissue repair. That stem cell detects damaged tissue as do the macrophages. These cleanup cells get rid of the dead and dying cells and then the local stem cell divides to replace itself and to furnish a new cell to replace the dead one. What does that stem cell do next?
Differentiation
The local stem cell that’s there to replace the dead tissue detects what type of cell it needs to be and then turns into (differentiates) that cell type (1). That cell is also usually primed in that direction anyway. That’s because the “stem cells” that live in these tissues are usually called progenitors as they tend to be more focused on becoming one cell type.
Natural Dedifferentiation
What if the local tissue is depleted of stem/progenitor cells? There’s a trick your body can do called dedifferentiation. That means it can go take cells backward from being a mature cell and turn them into a stem/progenitor cell. For example, see my video below on how that works in heart muscle:
The stem cells created by this process are usually of that cell lineage. For example, in the heart cell example above, the stem cells are cardiomyocyte progenitors (heart muscle stem cells).
Artificial Dedifferentiation
Can we take normal somatic cells and turn them into stem cells? Your body does it, so we should be able to make that happen in a lab. Turns out that there is a whole scientific discipline devoted to induced pluripotency or the chemical dedifferentiation of cells (iPSC or induced pluripotent stem cells) (2, 3). The cells created tend to be more pluripotent, meaning that they can turn into many different cell types, rather than the progenitors discussed above.
This technique is not without its difficulties. For example, the cells created often have genetic mutations (4). Why? the induced pluripotent stem cells created have all of the genetic mutation baggage of the older cells from which they were created.
Transdifferentiation
There’s another type of differentiation and it’s called transdifferentiation. This is where cells regress to a point when they can switch lineages. What does that mean?
Your cells have a lineage or family that they belong to just like you. That lineage is determined when they’re dividing in the embryo. At that point, they can become endoderm (inside), mesoderm (middle), or ectoderm (outside). For example, the mesoderm becomes your muscles, joints, and bones.
Transdifferentiation can take a cell of one lineage and convert it to another. For example, an endodermal cell can revert back to a stem cell and then switch to a mesodermal cell. This can happen naturally in situations where your body is trying to heal a skin wound (5).
The upshot? The fate of the body’s cells is fluid. Your stem cells can turn into needed cells that are damaged and when you don’t have enough of them, normal cells can revert back to stem cells. In addition, cells can also switch their family. Pretty crazy!
__________________________________
References:
(1) Almalki SG, Agrawal DK. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation. 2016;92(1-2):41-51. doi:10.1016/j.diff.2016.02.005
(2) Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16(2):115-130. doi:10.1038/nrd.2016.245
(3) Medvedev SP, Shevchenko AI, Zakian SM. Induced Pluripotent Stem Cells: Problems and Advantages when Applying them in Regenerative Medicine. Acta Naturae. 2010;2(2):18-28.
(4) Young MA, Larson DE, Sun CW, et al. Background mutations in parental cells account for most of the genetic heterogeneity of induced pluripotent stem cells. Cell Stem Cell. 2012;10(5):570-582. doi:10.1016/j.stem.2012.03.002
(5) Sayed N, Wong WT, Ospino F, et al. Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity. Circulation. 2015;131(3):300-309. doi:10.1161/CIRCULATIONAHA.113.007394

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.