A Review of the CharaCore Third Party Testing: An Altered Lab Report
I always say that you can’t make this stuff up. This past week I got sent what appeared to be independent third party testing on CharaCore, an umbilical cord product. After a little digging, it turns out the report isn’t worth the digital paper it’s written on. Let me explain.
What Is ChareCore?
I’ve blogged on Chara Biologics before, a cell therapy vendor started by a psychiatrist. Yep, you read that right, a psychiatrist. What would a psychiatrist have to do with stem cells? That’s a good question.
I had previously pointed out that the company’s claims that CharaCore has live and functional mesenchymal stem cells make it a drug, hence their 361 tissue registration wasn’t appropriate. After that blog, I got a nastygram from the company’s attorneys. Turns out the FDA agreed and sent CharaBiologics an Unititled letter, which basically said everything my blog did and then some. Hence, I was pretty sure that we wouldn’t hear too much more about CharaCore. Boy was I wrong.
Testing CharaCore
If you follow this blog, you’ve seen this image quite a bit. It’s the result of testing that was first performed in our lab here and then replicated at the CSU Translational Medicine Institute. This was on 5 umbilical cord products including CharaCore. While we tested many things that will be part of an upcoming publication, this is the “money shot”. White here means that no mesenchymal stem cells grew and purple means that there were stem cells. CharaCore is on the left and had no stem cells, while middle-aged and elderly bone marrow is on the right and had stem cells.
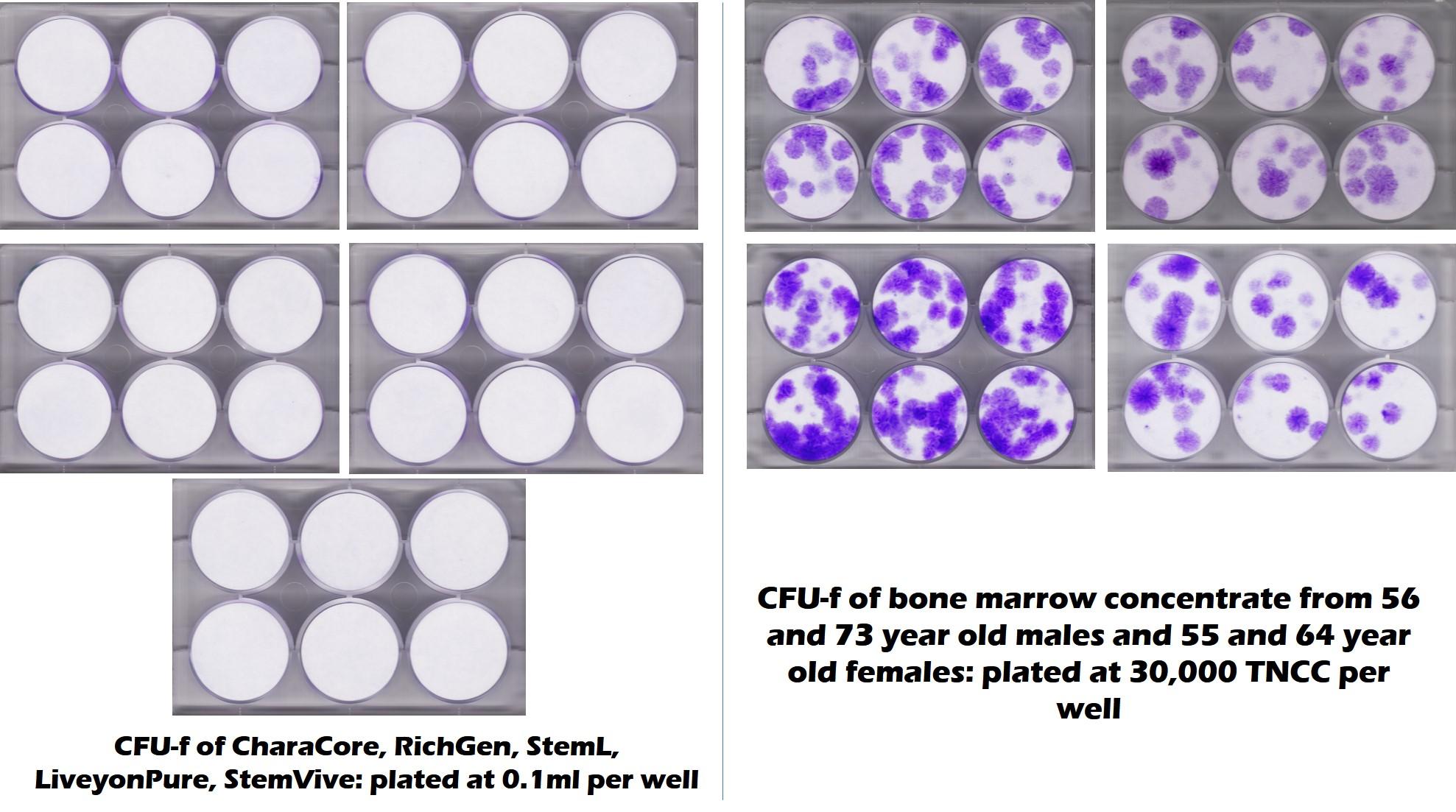
The New CharaCore Independent Third-Party Testing Results
I get sent stuff all the time. This past week, a colleague sent me a CharaCore report that included a bunch of regulatory language that I’ll cover in an upcoming blog. There was also what seemed to be independent testing on their product by a lab called “Creative Bioarray”. This is what that report looks like:

It seems to show that CharaCore has 12 million total cells with a viability of 85% and that about 20% of those cells are MSCs. In fact that 20% MSC content as compared to total nucleated cells is the first dead giveaway that something is very wrong here. Why? There is no tissue in anyone’s body that has a 20% MSC content. The richest adipose tissues have, as compared to the total cells present, an MSC content of usually less than 1%. Even fetal tissues including Wharton’s Jelly don’t approach 20% MSC content. In addition, the simple centrifuge steps that you would be allowed as part of minimal manipulation don’t concentrate MSCs relative to nucleated cells.
There’s also a whole section on how these cells apparently grew out to high CFU numbers (the CSU TMI test above). So how did we get from what we found above and on other tests to this report? The two independent tests couldn’t be more different. The answer to that question involves a little creative “alteration” of the Creative Bioarray report.
The Altered Creative Bioarray Report
When I looked at the Creative Bioarray report, since I’m involved with this data all day, lots of things didn’t look right. For example, flow markers that should be absent simultaneously had different values when they should be the same. The CFU numbers reported should vary widely by seeding density, but were basically the same. In addition, lots of supporting data and documentation that should have been in the report were missing. Hence, I contacted the company.
I asked several questions about the report and here are their responses in order:
- “We were shocked reviewing the document you sent over! We have indeed done such a project back in August, but the data in the document you sent over are completely different from what we have in our record! However, we cannot send the original reports to you due to the agreement between the client and our company.”
- I was fine with not seeing the original report, so I asked about the viability and CFU numbers-“Unfortunately, that’s not what we concluded. The number of live cells per vial and their viability were much lower. The CFU test result was far below 99… I feel uncomfortable reading the numbers shown in the document because they failed to reflect the original data we obtained.”
- I then asked about the fact that the report concludes that 20% of the cells were MSCs-No, that’s not the wording of our report. Our conclusion read “The cells do not meet the minimal criteria of hMSCs.” In the report, we also concluded that the cells showed hepatic and fibroblastic differentiation. To be honest, I would suggest that you do not believe any numbers shown in that document. When I said they were completely different from our record, I meant “completely different”.
Hence, the report I received and have archived and the one that is making the rounds between sales reps and physicians has been extensively altered to make it appear like the product contains MSCs.
Other Cases of Altered Reports
Everyone I tell this story to is shocked. However, they’re even more blown away when I tell them that this isn’t the first time I have documented this issue. If you’re an avid reader of this blog, you may remember when I found that a report being circulated by Predictive Biotech that had also been altered. The original report authored by the University of Utah claimed nothing about MSCs, while the report I received appeared to show that the product (CoreCyte) had loads of MSCs.
As I always say, you can’t make this stuff up! I am blown away that someone would do something like this. Was this done by the company? A sales rep? Someone outside the company? I don’t know, but I can say that I stand by the CSU tests of CharaCore that show that it does not contain viable and functional MSCs. That conclusion is also supported by emails from Creative Bioarray.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.