Direct Biologics, Exosomes, IRB Approval, and Paisleys?
What do Paisleys and illegal exosome products have in common? How about spine surgeons and exosomes? Let’s dig in.
Paisleys?
I recently got a press release that looks like it was put out by former Spine Surgeon, Ken Pettine. I’ve known Ken for years, but these past few years he’s suffered from an addiction problem and sadly lost his license to practice medicine. He used to be involved in a bone marrow concentrate clinic north of our Colorado HQ and had published some interesting research on the treatment of DDD through intradiscal injections along with Matt Murphy, Ph.D. This is the gist of the press release:
“Kenneth Pettine is co-founder of Paisley Laboratories and a co-developer of a bone marrow-derived mesenchymal stem cell active growth factor and exosome product that is anticipated to revolutionize regenerative medicine.
In this study, Extracellular Vesicle Isolate Product (EVIP) was injected into 33 retired Navy SEALs to assist with knee, shoulder, elbow, ankle, and wrist osteoarthritis. At three-month follow-up, the injection appeared both safe and effective, with improvements ranging from 40% to as high as 98%. The average improvement is over 70%.”
So let’s unpack this statement. Ken is the co-founder of something called “Paisley Laboratories”. What’s that? From online research, it’s a joint venture between Ken and the chief scientist from Direct Biologics. There is also a “Ken Allen” listed in the Paisley corporate records, but a reader tells me that this is Pettine’s middle name so that this is also Ken Pettine.
Next, we see Paisley produces an exosome product that was used in various joints of patients and three-month results of some sort are presented. Ken’s Linkedin profile also says that he is a consultant for Direct Biologics.
Exosomes Produced Via Culture Expansion Are an Unapproved and Illegal Drug Product
First, what the heck is an exosome? See my video below:
Exosome products have exploded in use, taking the integrative medicine and age management spaces by storm. However, are they legal? Let’s see.
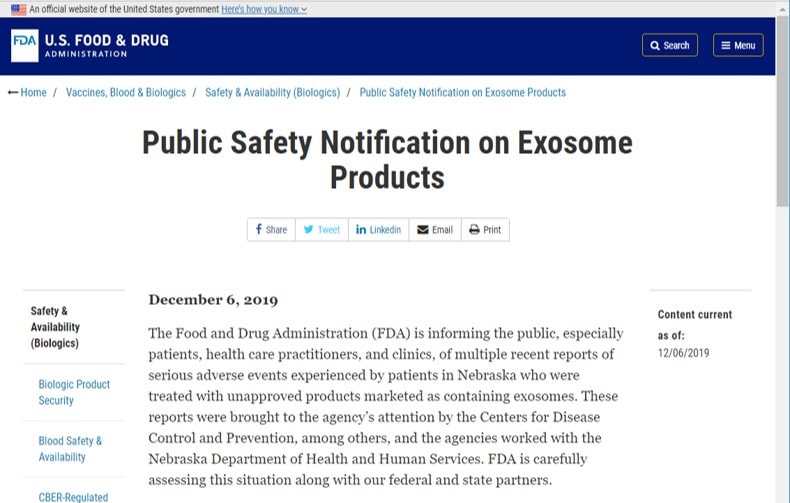
The FDA recently made no bones about the fact that it was nipping this whole exosome thing in the bud. They put out a rare public warning on the topic way quicker than they got in front of the whole fake umbilical cord “stem cell” debacle. Below is that public warning.
Hence the FDA made it very clear that any product claiming to have exosomes was a drug that required approval. So let’s vet the Pettine press release with this information.
Paisley Labs versus the FDA Regulations
Paisley clearly conducted some sort of study or treatment using exosomes that were derived from cultured cells. Is that legal? Not according to the FDA. In order to test a new drug (which is what these exosomes would clearly be classified as), you need an FDA study approval. That’s called a Biologics License Application/Investigational New Drug or BLA/IND. Basically you need to meet with FDA, tell them what you want to do, pay about a million USD to get enough pre-clinical data (or more) to have them approve a phase 1 trial. Did Paisley do that? It seems they skipped that step.
I reached out to Ken Pettine who is listed as one of Paisley’s founders and asked if Paisley had any of the above FDA approvals. The answer was clear that no such approvals were obtained.
Why None of this is Surprising
Listed on the Paisley website is the fact that Direct Biologics reports that they purchased the company. Who is Direct Biologics? A medical distributor that has been selling exosome products for quite some time. In fact, here they are at the American Academy of Anti-Aging Conference in Las Vegas this past December, just a week after the FDA announcement:
Realize that when I took this picture, the FDA had just made it crystal clear that the Direct Biologics 361 tissue registration that they had for this product was not sufficient. That meant that this booth advertising exosomes for clinical use shouldn’t be here. Don’t worry, Direct Biologics was only one of a number of companies at that conference exhibiting exosome products without FDA approval.
So the fact that Direct Biologics bought a company that is manufacturing exosomes and that these are being used outside of an FDA trial is not the least bit of a shocker. Remember that last year Direct Biologics was using a cadre of Colorado physicians in some type of network marketing plan for exosomes. In fact, online data states that Tim Mosely, Ph.D. who is the chief science officer for Direct Biologics was the other founder of Paisley.
Is an IRB Approval Enough?
One of the great refrains of the past decade in illegal biologics that require FDA approval is that the manufacturers or doctors using them claim that they have an IRB approval. This is a panel of experts who oversee research, but the problem here is that you need both an IRB approval and a BLA/IND FDA approval in place. Having just an IRB approval is like saying that you have a registration for your car, but no license to drive it, meaning it’s not enough. That same theme song is being played here as when I reached out to Ken Pettine he told me they had an IRB in place, but ghosted me when I asked about the FDA approval part (IND/BLA). I did have a back and forth email discussion with “Ken Allen”, who is also listed as a principal at Paisley, who seemed to lack understanding of the regulatory requirements of this type of product. This was really bizarre, as a reader tells me that “Ken Pettine” and “Ken Allen” are one and the same person, with Pettine now using his middle name as his last name.
The upshot? I often say that you can’t make this stuff up. Here we have a company that is growing stem cells and making exosomes which is clearly a drug product that requires FDA approval and that’s now using that product in human studies without any clearance from the FDA. Do exosomes work? Who knows. Are they any better than stem cells or PRP? Who knows. What I do know is that the FDA has made it crystal clear that exosomes are a drug that requires approval! In this case, that means you need an FDA BLA/IND BEFORE a clinical study can be conducted.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.