Liveyon Keeps Misleading Physicians
Liveyon has been featured here many times. Frankly, after the recent podcast called “Bad Batch” I was pretty sure that they would fade into the sunset. However, a recent marketing email shows that they are alive and well and continue to deceive doctors.
What is Liveyon?
Liveyon is a company that used to distribute an umbilical cord blood product manufactured by Genetech (not to be confused with the pharma company called Genentech). That lead to a contaminated product which placed many people in the ICU. To learn more in-depth about that, see the Wondery podcast below called “Bad Batch” (click on the pic to hear the podcast):
The company then decided to manufacture its own umbilical cord product, called Liveyon Pure. Despite receiving an FDA Warning Letter about the claims it made of selling stem cells for it’s contaminated Regen Series product, the company still claims to sell a stem cell product on its website today. In fact, there is its medical director, podiatrist Allan Galvec, in a video that begins with a nude blonde set on a black background, claiming that Liveyon sells stem cells:
The Sales Pitch
This is an email recently sent to a colleague who forwarded it onto me:
“My name is JP Webb and I am with LIVEYON LABS. I wanted to send you some information about the MSC products we have for your regenerative medicine patients. If this is of interest to you, I would like to set up a time to come and discuss how our MSC products would be a good compliment to your regenerative medicine clinic… ”
What is an “MSC” product? That’s an abbreviation for Mesenchymal Stem Cell. Hence, this email is claiming that the Lioveyon PURE product has MSCs.
I called JP, who just started as a sales rep with Liveyon. He was completely unaware that claiming that Liveyon had “MSCs” was not regulatory compliant. He also didn’t understand any of the science behind what he had sent. When I asked for instance about CD73, his response was basically that he was sending the sales pitch that Liveyon had told him to send.
CD 73? Really?
You almost can’t make this one up. The email also included this claim:
“Mesenchymal Stem Cell (MSC) content of 3%, per CD73 marker”
What scientist is advising these guys? CD73 is a white blood cell marker (leukocyte) that is also found on MSCs. However, you also need multiple other markers present and absent on those MSCs to make the call that they are a stem cell. Think of it this way. You’re looking for a new car and you want a red Mercedes SL 500 convertible. You arrive at the car lot and you tell the salesman what you want and he says, “You bet, we have 100 red cars”. You look at him strangely and again repeat that you’re not looking for a red car, but a specific red Mercedes SL. He again repeats that they have loads of red cars.
What I just recounted with the car lot and the off-kilter salesperson is the same as saying that that the cells have the CD73 marker and are therefore stem cells. MSCs need to have many more markers that should be there including CD73. So like our red Mercedes SL 500, there are many properties that define that stem cell type. So the Liveyon email is like the used car salesman here, just repeating that they have loads of cells with CD73, some of which may or may not be the cell you’re looking for.
Playing with Flow Cytometry for Fun and Profit
Most physicians have no idea what flow cytometry data means in that they don’t see it every day and it isn’t part of usual practice. In addition, a major market for Liveyon (as discussed in the Podcast above) is chiropractors, who are even less likely to know which end is up with flow cytometry. Hence, when Liveyon or any other birth tissue company reports flow data, it’s often misinterpreted or subtly not well interpreted for its own purposes.
You know from my description of the red Mercedes SL 500 that in order to describe a stem cell you need a bunch of markers to be present and absent. This again is just like the car we want. It has to be red and not green. It has to be a convertible and not a Coupe. A Mercedes and not a Porsche. Etc… Hence, describing a thing (like a stem cell) by its properties needs to report stuff that is and isn’t there.
You know from above that CD73 is just one of many markers, like saying that the dealer has lots of red cars. Liveyon in the above email also included a document entitled, “WhitePaper_061v1 Comparison of Birth Tissue Products”. In this document, Liveyon says that it tested the flow cytometry markers CD19, CD34, CD45, CD73, and CD90. Again, this is like saying that we have cars that are red, are not Coupes, are convertibles, are not Porsches, are Mercedes, are SL500’s etc… However, to find the red Mercedes SL500 convertible, it needs to have all of those properties and be missing others. In the case of these flow markers, a mesenchymal stem cell would need to have CD73 and CD90 present, plus CD105 that wasn’t tested here. The other markers would all need to be absent. That was never reported by Liveyon, hence nothing in this document supports that there are MSCs in the product. To learn more about flow cytometry, see my video below:
Actual Tests of Liveyon Pure
Below is an actual CFU-f test conducted by the CSU Translational Medicine Institute on multiple umbilical cord products, including Liveyon Pure:
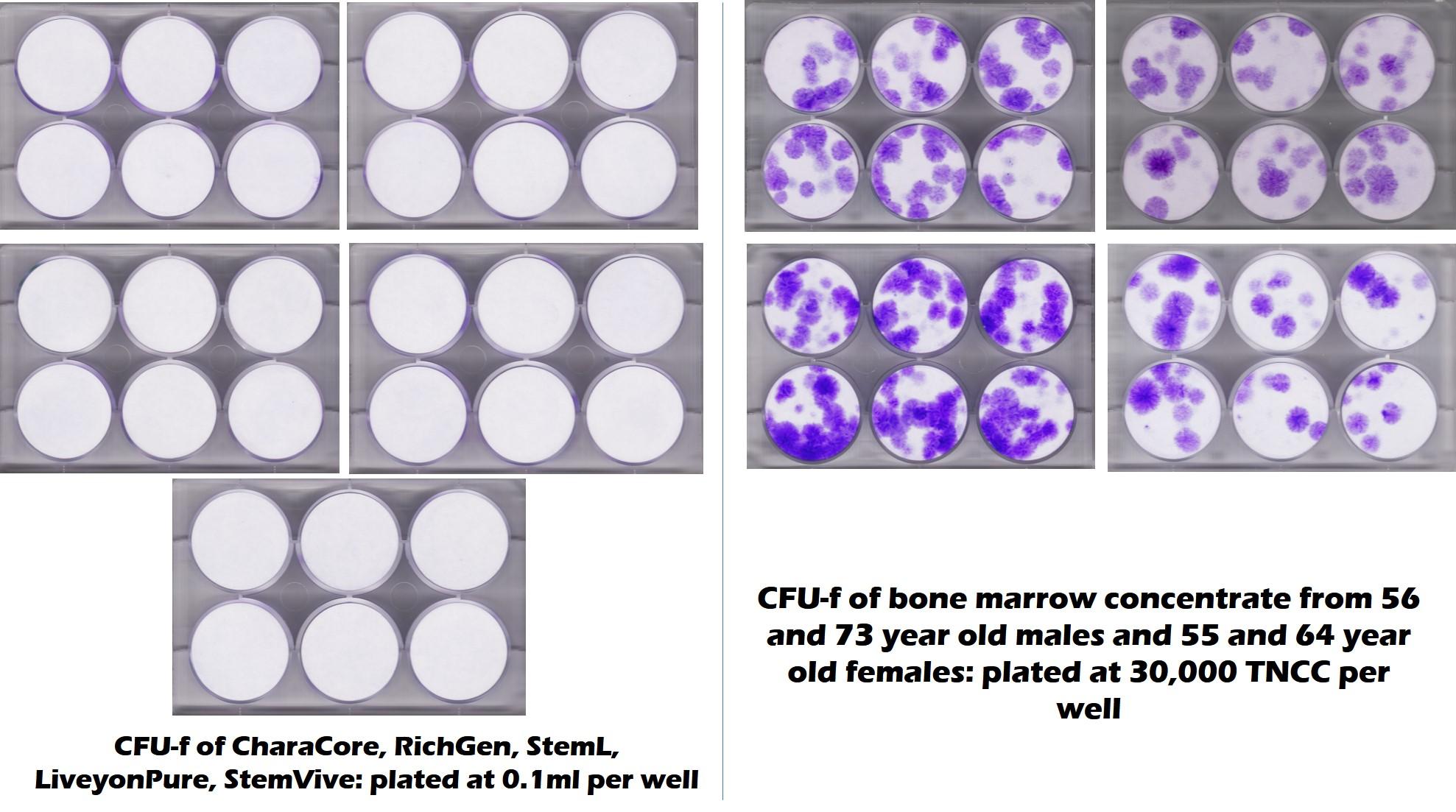
The purple dots to the right represent the stem cell content of middle-aged and elderly bone marrow. The all-white that you see on the left represents no stem cells in Liveyon Pure and other products. Similar tests at our lab also got the same result:
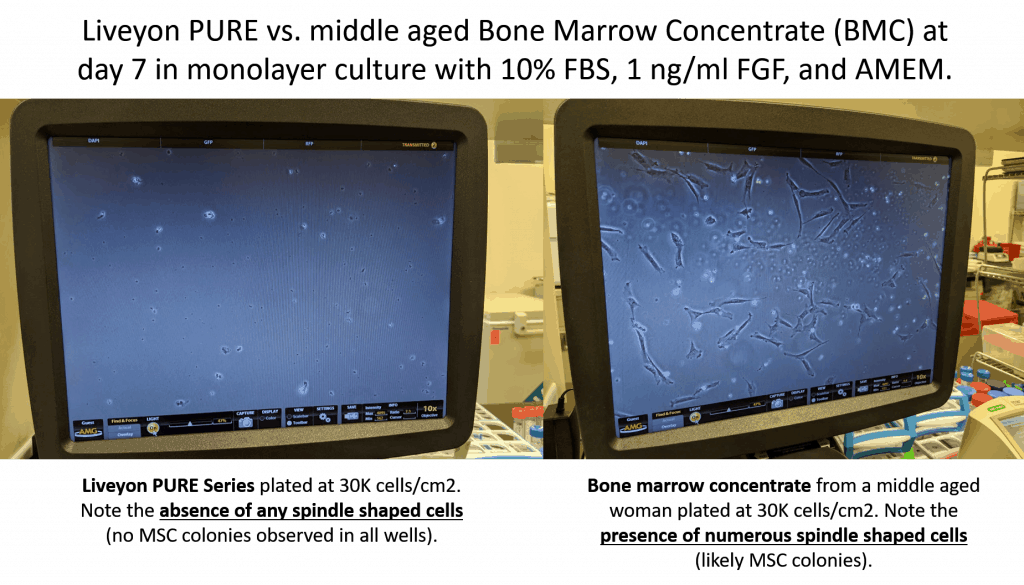
The upshot? Liveyon on its website still claims that it sells stem cells. It’s marketing e-mail claims that it’s selling MSCs. Hence, you would expect that the flow cytometry data would show that the product had MSCs. Close, but no cigar. Meaning the flow data doesn’t show anything of the sort. In fact, independent tests show no live and functional MSCs. Hence, Liveyon continues to mislead physicians.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
