Predictive Biotech and an EUA for CoreCyte?
One company that I have tracked extensively here on this blog is Predictive Biotech. It sells a product that it claims contains mesenchymal stem cells (MSCs) and has now submitted an Emergency use Authorization to the FDA to use this product to treat COVID-19. The rationale is that the product has loads of MSCs. The problem? It’s very unlikely that this product contains any living or viable MSCs.
The Research on Using MSCs for COVID-19
There has been a handful of promising small studies that suggest that mesenchymal stem cells (MSCs) could help patients who are in the ICU with COVID-19 (1). For example, one small study was published on severe patients who were given 50-100 million, culture-expanded MSCs. In particular, these are cells that are isolated and grown in a lab and are not available to use here in the U.S. These MSCs were genetically modified to be focused on the cell receptor that the virus attacks.
An FDA EUA?
The FDA has issued several Emergency Use Authorizations or EUAs. These are special approvals for things that may help in the coronavirus crisis. I was really surprised the other day to see this press release from Predictive:

Why? The product that Predictive claims to be using is called CoreCyte. The issue is that this product likely contains no living and functional mesenchymal stem cells. So how could Predictive put out a press release claiming that it applied to FDA to get an EUA for a stem cell product without stem cells?
Predictive’s FDA Compliance
First, Predictive Biotech is a small company in Utah that sells birth tissue products. Contrary to FDA guidelines, it has been claiming that these products contain mesenchymal stem cells and is selling these products using a simple tissue registration that takes 45 minutes online rather than obtaining full FDA approval. The FDA has stated in this general announcement that companies claiming to sell stem cell products and registering themselves under the tissue registration pathway described above aren’t legal:
That FDA problem hasn’t stopped Predictive from aggressively advertising that it’s selling stem cells.
Umbilical Cord Products and Stem Cell Claims
Predictive isn’t the only company playing what, in my opinion, is a stem cell shell game. In fact, a number of companies have been playing this same game. This means that they’ve been selling various components of the umbilical cord or birth tissues and claiming that these are stem cell products. The issue is that many labs, including ours, the CSU Translational Medicine Institute, Cornell, and UC Davis have independently tested several of these products and found that they contain no living and functional stem cells (2-4).
In fact, since a picture is worth a thousand words, here’s one from an upcoming publication by our team and the CSU Translational Medicine Institue on these products:
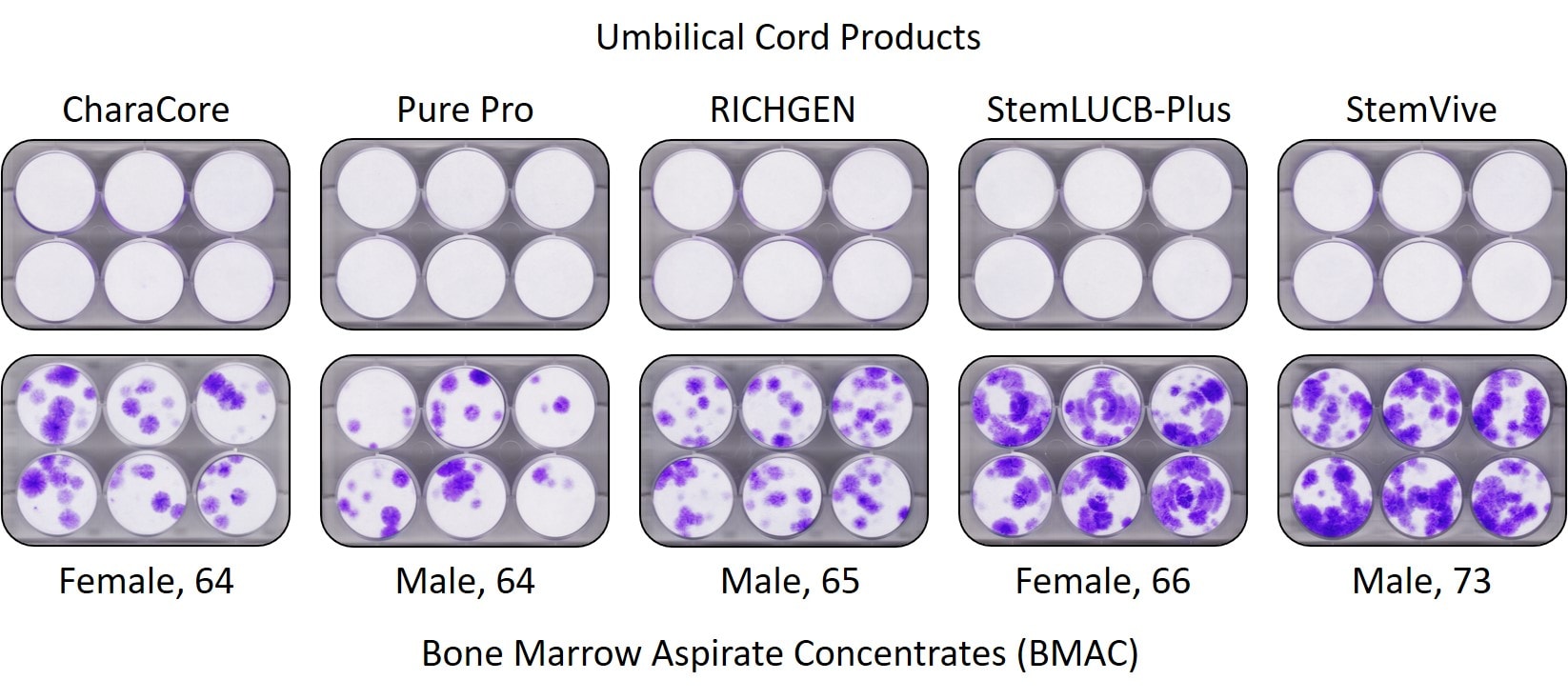
The purple dots in this CFU-f test represent stem cell colonies. The umbilical cord products being sold as containing many young and highly active stem cells are across the top. That sterile white you see in those plates means that they contain no living and functional mesenchymal stem cells. The bottom row is the stem cell content of bone marrow from the elderly, which contains plenty of stem cells. Why include bone marrow from old people here? Because these companies selling this stuff often claim that older people have so few stem cells that the reason a doctor should pay 1-2 thousand dollars a vial for their birth tissue stem cell product is that it contains many, many more. The truth is, of course, the opposite.
CoreCyte?
The press release above says, “The proposed IND clinical trial will utilize Predictive’s proprietary core technology of naturally occurring MSCs derived from umbilical cord tissue (UC-MSCs) to assess the efficacy as an add-on therapy to standard treatment of patients with severe Acute Respiratory Distress Syndrome (ARDS) secondary to COVID-19.” The press release then states that the product Predictive will use is Corecyte.
Corecyte contains Wharton’s Jelly derived from a baby’s umbilical cord as most of the products shown above do. Given that Predictive can only do so many things to that product, in my opinion, it’s VERY UNLIKELY that CoreCyte has any viable and functional mesenchymal stem cells. In fact, we tried to get CoreCyte vials from Predictive, but, after several rounds of back and forth discussions the company never permitted our lab or CSU to test its product.
You Can’t Make This Stuff Up
So to review, we have:
- A company that has skirted FDA regulations for years by claiming that it’s selling a stem cell product (CoreCyte) and instead of getting an actual FDA approval, has been misregistering that product as a tissue without any FDA approval.
- That product, CoreCyte, based on the peer-reviewed published and presented literature to date on similar products likely has no viable mesenchymal stem cell content.
- The company just put out a press release claiming that it’s CoreCyte product should work for COVID-19 induced ARDS because it has mesenchymal stem cells and those were shown to work in the study referenced above (which didn’t use this same product, but instead mesenchymal stem cells that were grown in a lab with an artificial gene inserted).
Huh? While the company is free to throw its proverbial hat in the ring and try it’s umbilical cord tissue product in ARDS patients in the ICU if the FDA believes that’s safe, claiming that its product should work because it has loads of MSCs when it likely has no viable and functional stem cells, is in my opinion, theater of the absurd.
The upshot? The coronavirus crisis is bringing out some weird stuff. In my opinion, this one is really crazy. Hopefully, cooler heads at FDA read this press release.
[Note, unless your email address can be verified as real and in current use as a primary email address, we will not post comments.]
_____________________________________
(1) Leng Z et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging and Disease. 2020, 0 (0): 216-228 https://doi.org/10.14336/AD.2020.0228
(2) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(3) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(4) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
