The Facebook Group Misinformation Saga Continues
If you read this blog, you know that I smoked out one Facebook group on stem cells that was really a sales funnel for a clinic referral service and identified another one that’s run by patients full of sales reps and paid promoters where patients are getting bad information. Can you fix the stream of misinformation?
Patient Discussion Groups
Patient discussion groups, whether online or on Facebook are a great resource. A loved one of mine needs surgery. I don’t know much about that procedure and I intend to peruse many groups to get information directly from patients about their experiences. So even doctors can get something out of these groups.
However, as I encouraged my family member to join some of these groups himself, I warned him about paid clinic promoters. Being a millennial, he sort of knew at face value that these groups might be filled with salespeople. Having said that, I’m not sure that most patients know that the person who responds to their post who looks like a fellow sufferer, may be paid to push a product or clinic.
One group that has a problem with paid promoters is a Facebook Group called Stem Cells and Exosomes. It’s filled with patients who are just trying to learn, and run by some patients who are trying hard to help, but it’s become a mecca for misinformation. I joined the group as an experiment to see if I could clean this up. I lasted about a week as the paid promoters complained to the admins that I was wrecking their tenuous sales pitches. However, one of the group members reached out to see if I would respond to the posts of the paid promoters who are now trying to clean up the mess I caused by bursting their sales bubbles. Hence, today I’ll post the sales rep comments from the group and then my responses. Hopefully, someone can get these posted in the group.
The Sales Rep Posts
Here is the post I was asked to respond to:
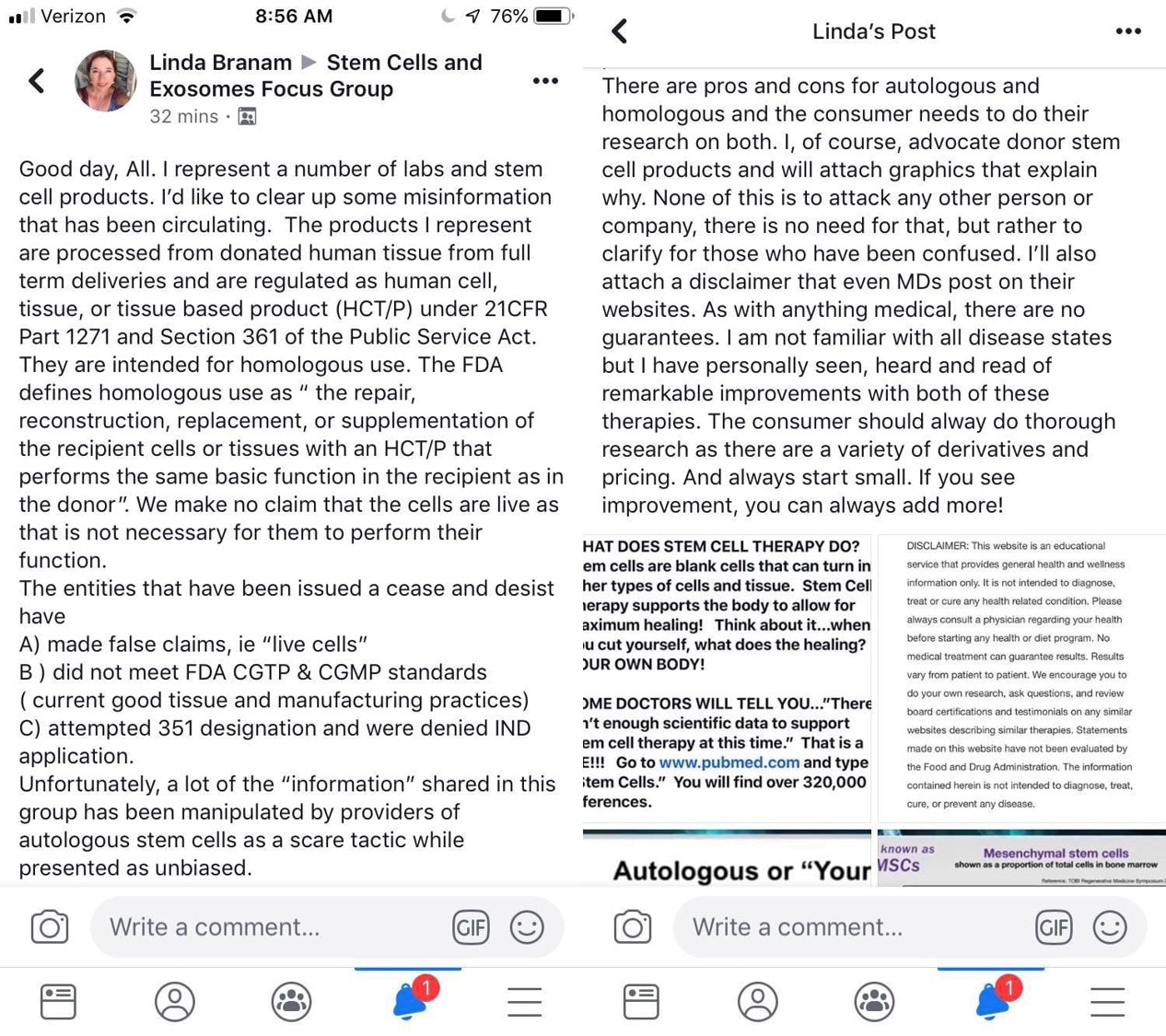
This was posted because I called Linda out for answering a patient’s question about her husband’s Erectile Dysfunction. Linda sells a Wharton’s Jelly umbilical cord product and exosomes. She claimed that these products could cure this poor woman’s husband’s ED and that there was lots of research to prove that. I called Linda out by actually posting real research studies, but none of those studies used her employer’s products or even Wharton’s Jelly or exosomes. So let’s break down the truth from fiction in Linda’s new posts.
First, let’s explore what Linda sells. Let’s look at Linda’s information online:
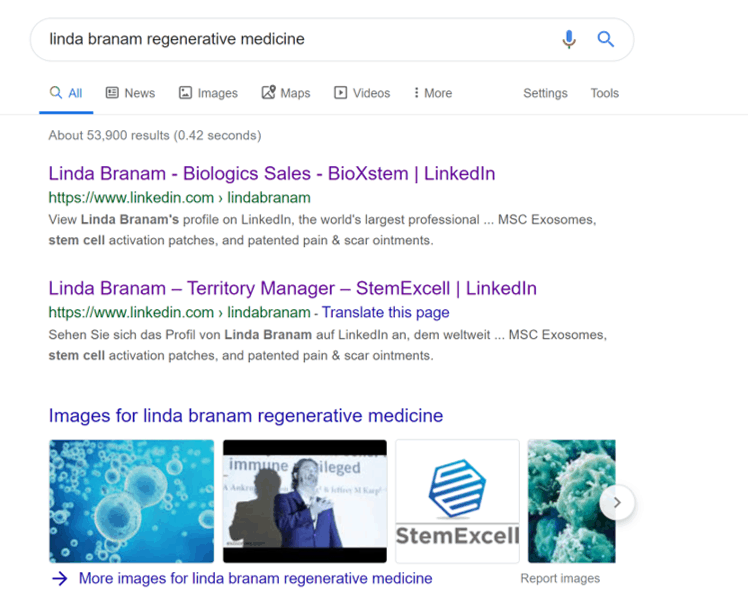
Linda’s two Linkedin profiles are filled with claims about selling MSCs and exosomes. She’s linked to a medical sales company called StemExcell. I’ve come across the aggressive claims of this company before as they were pushing exosomes for Kimera Labs (which has sales reps and company reps on this Facebook group). On the StemExcell Facebook page, we see a video where the CEO of Kimera (Duncan Ross, Ph.D.) makes health claims that the exosomes can treat burns, MS, hair loss, and renal dysfunction to name a few. We also know that FDA considers exosomes a drug that can’t be registered as a donor tissue (361), but must undergo extensive clinical trials for each clinical application (351). These are responses from the FDA TRIP program on exosomes and Wharton’s Jelly products:


Both of these letters show that these products can’t be registered as a 361 donor tissue as Linda states, but instead must go through full FDA approval after clinical trials for each medical indication. In addition, multiple FDA Warning Letters including this one show that claims of selling MSCs will not be permitted as a 361 donor tissue. So let’s see if everything else Linda said is true:
- What she sells is properly registered as a 361 donor tissue. Nope, both MSCs and exosomes have been called out by FDA as requiring clinical trials and full FDA approval before use and NOT a simple 361 online tissue registration.
- That what she sells is “homologous use”. The Stemell letter and others say specifically that umbilical cord products used to treat orthopedic or non-cancer indications are NOT homologous use. This means that the FDA considers them to be used differently in the body than these applications, so another way to say it is that these cells don’t naturally repair orthopedic or other diseases in adults.
- StemExcell makes no claims that the cells are alive as that’s not necessary for them to perform their function. Stem cells work through either differentiation or a paracrine function (13). This means either they become another cell that’s in need of replacement/repair or they excrete growth factors and other things to help repair. Dead cells don’t do either. Is there any published clinical data that the Wharton’s Jelly product or the Kimera exosome product that Linda sells is effective for any clinical indication? None that I have found.
- Looks like I got a cease and desist letters from someone’s attorney? So that you understand, anybody can send anybody else a cease and desist letter. Generally, that’s part of a SLAPP strategy or threatening a lawsuit to inhibit public discourse. If so, I’ll add it to my wall of fame, as stating the truth will get you those, but I’m firmly protected by the first amendment and the best defense is the truth. Not sure what that whole A,B,C paragraph stuff is all about, as this is not based on anything I wrote in this Facebook group.
- Disclaimers are all she needs. Nope. You can’t make a claim that you’re selling MSCs or exosomes without an FDA approval for same and then throw a disclaimer up.
Digging into the Information that She Posted
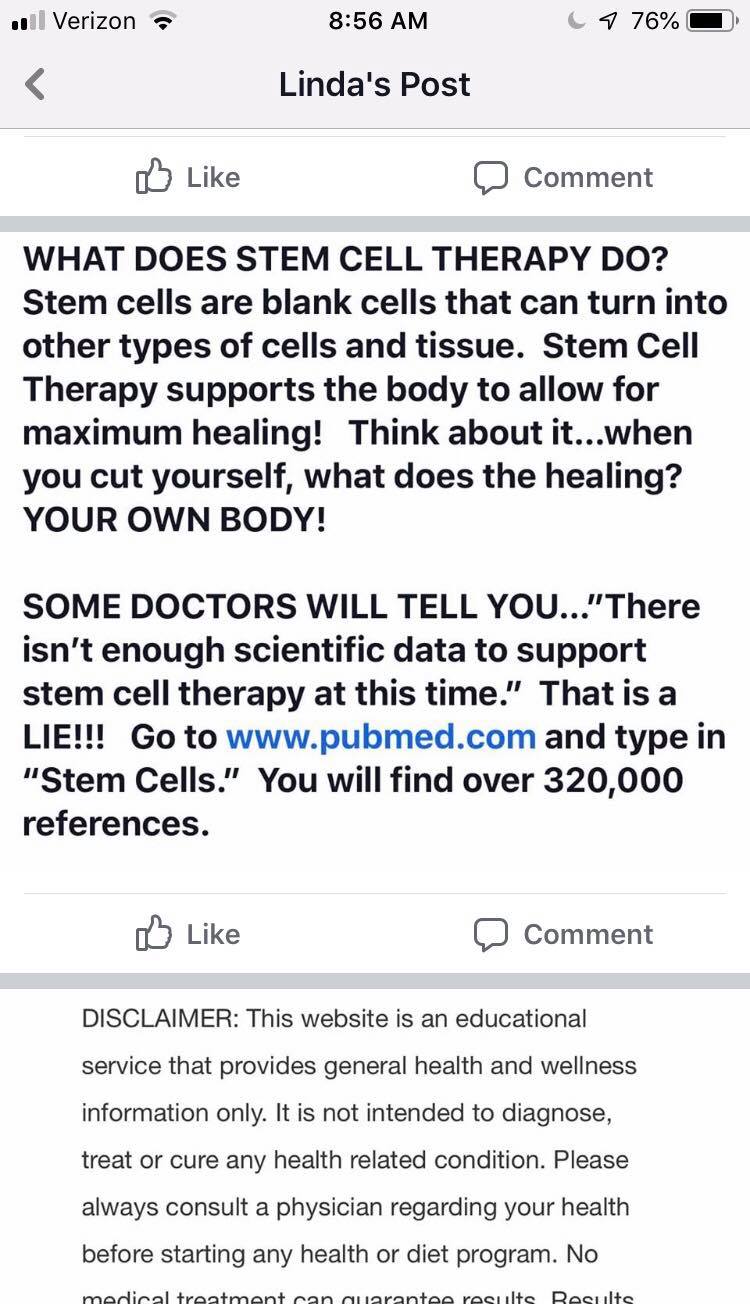
OK, Linda just told us that she didn’t sell live stem cells or made no claims that the stem cells she sells are living. That reminds me of my dead Goldfish video below (it’s about amniotic, but it applies to Linda’s dead umbilical cord cells as well):
Here Linda makes several health claims that would make her Wharton’s Jelly product an unapproved drug:
- Stem cells (presumably the one she sells) can heal the body.
- There are 320,000 PubMed references for stem cells
The problem here is that while stem cells in early clinical trials (like this one we published (14)) can likely help the body heal, nobody has ever tested Linda’s products to see if they work in clinical trials. So of that 320,000 articles on stem cells, NONE is a published clinical trial using the Wharton’s Jelly or Kimera exosome product on which she earns commissions. In addition, any clinical trials in that 320K that use stem cells, use living stem cells, often after isolation and culture expansion.
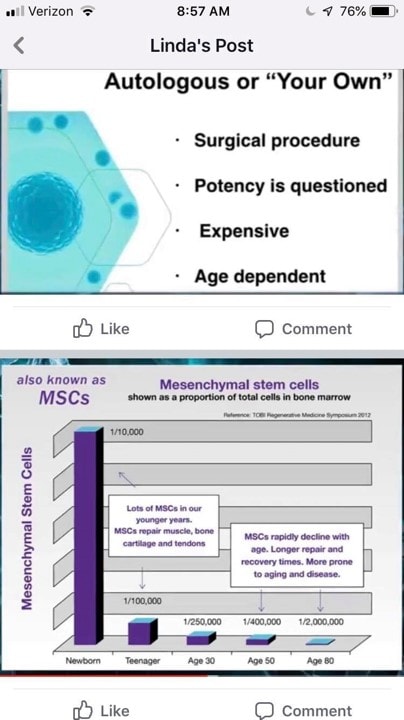
Let’s now take the rest of this and dissect it:
Autologous Cell Therapy is Invasive
First, Linda is talking about a Bone Marrow Aspiration, which is how stem cells are harvested. Is this a surgical procedure? It’s actually more like a fancy blood draw. Why? As far as safety is concerned, in the largest safety study published on BMA there were 15 complications in 20,323 procedures (1). That’s a complication rate of 1 in 1,355 procedures. These were minor complications. So if a BMA is surgical, it’s an awfully safe procedure. Meaning for comparison, the rate of complications for arthroscopic knee surgery would be 1-8 in 100 (2). Spine surgery has a complication rate of 16 in 100 (3).
How safe are allogenic umbilical cord products when used to treat orthopedic and other incurable non-cancer diseases? There is no large scale research study to quote. We do know that many need to be HLA matched and aren’t, which risks Graft vs. Host Disease (GVHD) (4-7). We also know that while it’s been thought that living stem cells can inhibit the host’s immune response to foreign cells, recent research has shown that’s not completely true (12). In addition, dead stem cells are more dangerous than live ones, as dead cells can’t inhibit the host’s immune system (see GVHD discussion above). We also know of various stem cell contamination cases where many patients ended up in the ICU (8). So which is safer?
Potency is Questioned?
Not even sure how to answer this one as we have dozens of clinical studies performed with autologous stem cells that show efficacy. In fact, here’s a list of those just in orthopedics with a link to each study:
Again, we have no clinical trial published that uses Linda’s Wharton’s Jelly or exosomes products.
Expensive
Most of the providers I know who are performing umbilical cord treatments are charging about the same or more than what we charge for autologous therapy. You can certainly get a deal if you go to certain offices that use a lesser trained nurse with limited expertise to perform the procedure. However, in general, both procedure types cost about the same, near as I can tell.
Age-Dependent
Here we see the slide used in every local chiropractic presentation on stem cells. It seems to show that the number of stem cells in the bone marrow drops precipitously with age. However, that’s not entirely accurate. Let’s dig in.
What Happens to Stem Cells as We Age?
First, you have millions of stem cells LIVING in your body RIGHT NOW (15,17,18). They are the day to day repairmen of your tissues, so if you’re alive and kicking, they are functioning fine. Meaning that if we wiped out all the stem cells that live in your bone marrow, you would die in weeks to months (16,19).
Second, many have believed that stem cell number drops hugely with age, just as Linda’s slide shows above (20). However, our research team has performed the largest study to date examining the number of mesenchymal stem cells present in the bone marrow as a person ages. If you take away babies and kids and just focus on the adults, there isn’t a relationship between the number of stem cell cells in the bone marrow and age. This is shown in the diagrams below which are part of an upcoming research publication:
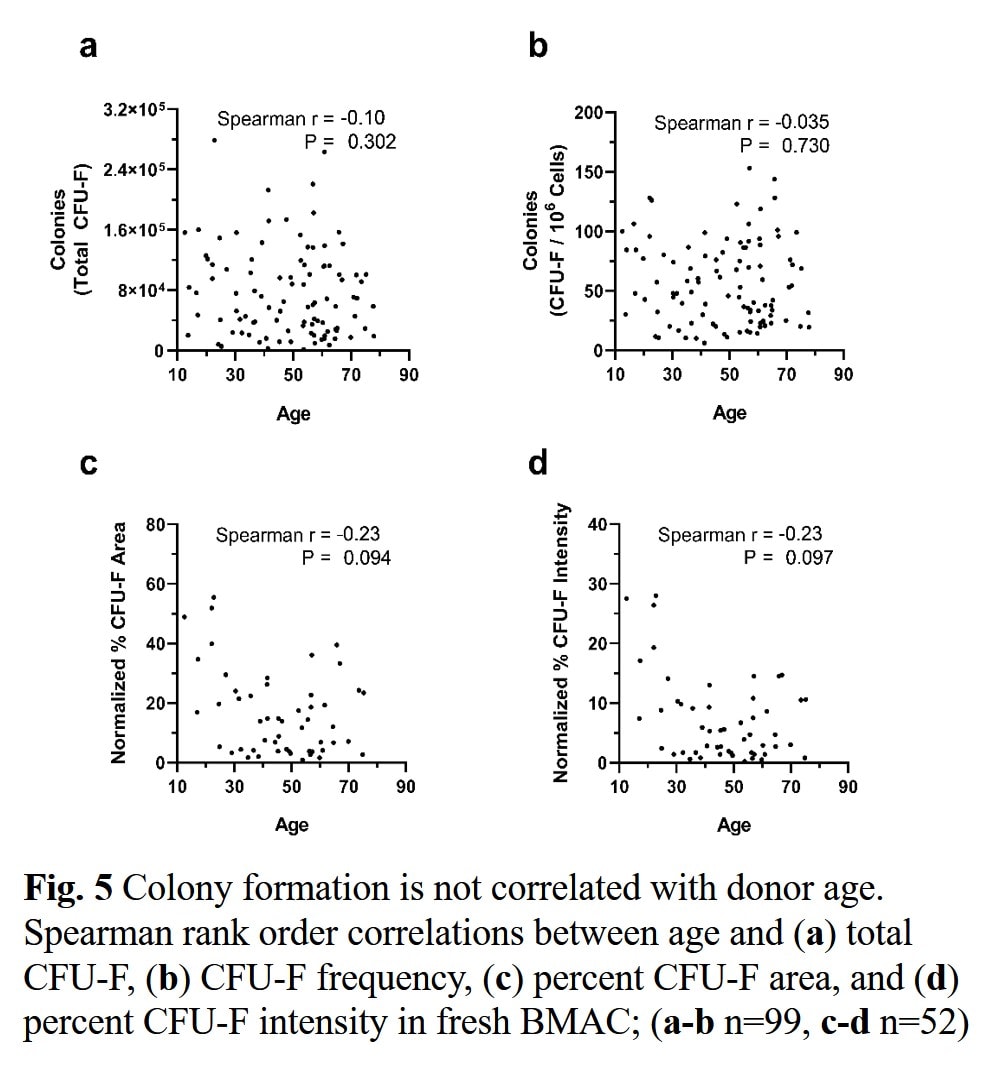
While this may look hard to understand, the graphs show no relationship between the stem cell numbers present in these bone marrow samples the patient’s age. How about the studies that did? They are smaller than the dataset shown above and many suffer from methodology problems in running the CFU-f analysis similar to those I described here.
Can You Be Too Old to Use Your Own Stem Cells?
I can answer that with some of our own published research papers. In one study on knee arthritis patients, we looked at more than 800 patients who had their own concentrated bone marrow stem cells injected (21). There was no significant relationship between their age and how they responded, meaning patients that were older didn’t do any worse than patients who were younger.
In another published research study, what we did find that caused less robust outcomes in older patients was a low dose of total bone marrow cells (which is also linked to a low total stem cell dose) (22). These poor results could be easily overcome by delivering more cells through a better bone marrow stem cell draw and then improved processing of the sample. Meaning, having older stem cells didn’t reduce a patient’s chance of success, you just need to concentrate their cells to a minimum level to see good results.
We’re not the only research group to find this interesting relationship between stem cell dose and outcome. Others have found this correlation when treating bones that won’t heal (fracture non-union) and painful low back discs (23, 24). Hence, getting autologous cell therapy to be effective in older patients is all about concentrating their cells.
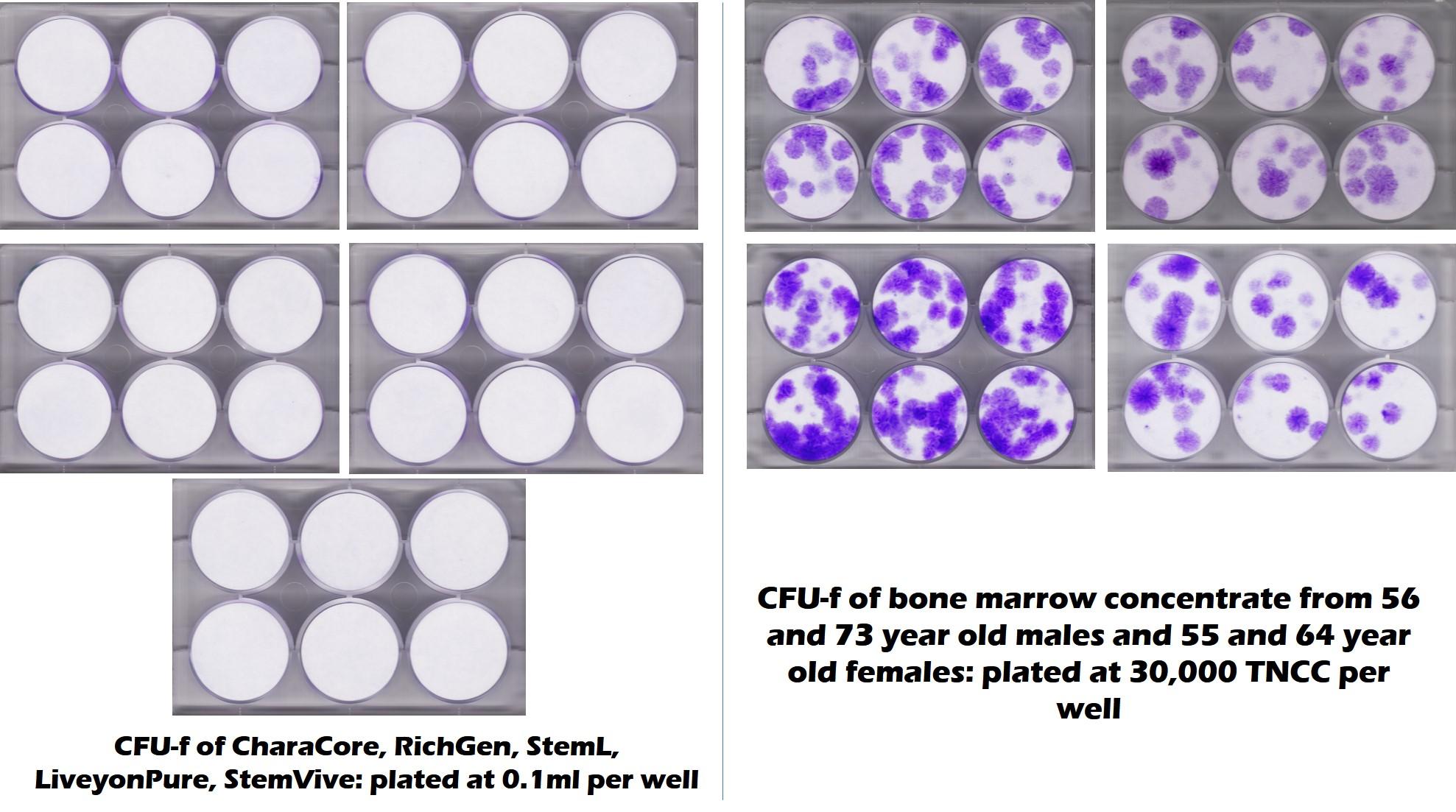
Umbilical Cord Products Have No live and Functional Stem Cells
A picture is worth a thousand words. The 6-well plates below are from a CFU-f analysis performed by the CSU Translational Medicine Institute. On the left, they tested 5 common umbilical cord stem cell products and on the right, they tested middle-aged and elderly bone marrow. The purple dots in this test represent stem cells and the blank white means no stem cells. On the left, the result was no living stem cells in all 5 commonly sold and used 361 registered umbilical cord products. On the right, we see the stem cells present in the older bone marrow. Others have found the same results in other birth tissue products used by clinics (9-11). Hence, Linda’s argument just went out the proverbial window.
Closing Thoughts
Here I am sitting on a plane heading back from my son’s college parent’s day. I will spend about 4-5 hours of my time today writing this response and another 2 hours proofing and citing it tomorrow at 5:30 am. Which is the problem with sales reps. They can put out lots of stuff that looks official or even scientific. Unless you’re highly knowledgeable about the regulations and the science in this area and up to date what’s coming out of the FDA, 99% of patients and physicians won’t know it’s inaccurate. In addition, it will take many hours of time to properly rebut.
The upshot? I was asked to rebut this sales rep’s statements and now that’s done. Enough with companies and sales reps pushing products that aren’t what they claim to be or aren’t permitted per existing FDA regulations. It’s exhausting!
____________________________________________
References:
(1) Bain BJ. Morbidity associated with bone marrow aspiration and trephine biopsy – a review of UK data for 2004. Haematologica. 2006 Sep;91(9):1293-4. https://www.ncbi.nlm.nih.gov/pubmed/16956842
(2) Friberger Pajalic K, Turkiewicz A, Englund M. Update on the risks of complications after knee arthroscopy. BMC Musculoskelet Disord. 2018;19(1):179. Published 2018 Jun 1. doi: 10.1186/s12891-018-2102-y
(3) Nasser R1, Yadla S, Maltenfort MG, Harrop JS, Anderson DG, Vaccaro AR, Sharan AD, Ratliff JK. Complications in spine surgery. J Neurosurg Spine. 2010 Aug;13(2):144-57. doi: 10.3171/2010.3.SPINE09369.
(4) Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood Jul 2014, 124 (3) 363-373; DOI: 10.1182/blood-2014-01-514786
(5) Lee SJ. Classification systems for chronic graft-versus-host disease. Blood Jan 2017, 129 (1) 30-37; DOI: 10.1182/blood-2016-07-686642
(6) Eapen, Mary et al. Mismatched Related and Unrelated Donors for Allogeneic Hematopoietic Cell Transplantation for Adults with Hematologic Malignancies. Biology of Blood and Marrow Transplantation, Volume 20, Issue 10, 1485 – 1492. https://www.ncbi.nlm.nih.gov/pubmed/24862638
(7) Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–1930. https://www.ncbi.nlm.nih.gov/pubmed/15191952
(8) (11) US Food and Drug Administration. “FDA sends warning to company for marketing dangerous unapproved stem cell products that put patients at risk and puts other stem cell firms, providers on notice” 12 August 2019, https://www.fda.gov/news-events/press-announcements/fda-sends-warning-company-marketing-dangerous-unapproved-stem-cell-products-put-patients-risk-and .
(9) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(10) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(11) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(12) Berglund AK, Fortier LA, Antczak DF, Schnabel LV. Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res Ther. 2017;8(1):288. Published 2017 Dec 22. doi: 10.1186/s13287-017-0742-8
(13) Zhang B, Luo Q, Halim A, Ju Y, Morita Y, Song G. Directed Differentiation and Paracrine Mechanisms of Mesenchymal Stem Cells: Potential Implications for Tendon Repair and Regeneration. Curr Stem Cell Res Ther. 2017;12(6):447-454. doi: 10.2174/1574888X12666170502102423.
(14) Centeno C, Sheinkop M, Dodson E, et al. A specific protocol of autologous bone marrow concentrate and platelet products versus exercise therapy for symptomatic knee osteoarthritis: a randomized controlled trial with 2 year follow-up. J Transl Med. 2018;16(1):355. Published 2018 Dec 13. doi:10.1186/s12967-018-1736-8
(15) Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. Published 2014 May 19. doi:10.3389/fimmu.2014.00148
(16) Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125. Published 2016 Aug 31. doi: 10.1186/s13287-016-0363-7
(17) Lee-Six, H., Øbro, N. F., Shepherd, M. S., Grossmann, S., Dawson, K., Belmonte, M. Campbell, P. J. Population dynamics of normal human blood inferred from somatic mutations. Nature 561, pages 473–478, 2018. doi:10.1038/s41586-018-0497-0
(18) Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells – current trends and future prospective. Biosci Rep. 2015;35(2):e00191. Published 2015 Apr 28. doi:10.1042/BSR20150025
(19) Green DE, Rubin CT. Consequences of irradiation on bone and marrow phenotypes, and its relation to disruption of hematopoietic precursors. Bone. 2014;63:87–94. doi:10.1016/j.bone.2014.02.018
(20) Ahmed AS, Sheng MH, Wasnik S, Baylink DJ, Lau KW. Effect of aging on stem cells. World J Exp Med. 2017;7(1):1–10. Published 2017 Feb 20. doi:10.5493/wjem.v7.i1.1
(21) Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int. 2014;2014:370621. doi:10.1155/2014/370621
(22) Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet Disord. 2015;16:258. Published 2015 Sep 18. doi:10.1186/s12891-015-0714-z
(23) Homma Y, Zimmermann G, Hernigou P. Cellular therapies for the treatment of non-union: the past, present and future. Injury. 2013 Jan;44 Suppl 1:S46-9. doi: 10.1016/S0020-1383(13)70011-1.
(24) Pettine KA, Suzuki RK, Sand TT, Murphy MB. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int Orthop. 2017 Oct;41(10):2097-2103. doi: 10.1007/s00264-017-3560-9.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.